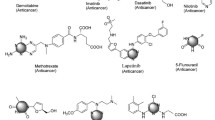
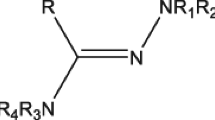
Cancer is a rapid, out of control, and proliferation pathology of ordinary cells, which has become one of the most infamous and aggressive health afflictions worldwide. Molecular hybridization is related to a combination of two or more pharmacophores of bioactive frames which generate a single molecular structure with enhanced activity. Previous studies described the piperazine framework as a central structure in numerous synthetic and natural compounds, showing a wide range of biological activities. Here, we review, highlight and discuss a detailed account of the anticancer applications of some important piperazine-containing hybrid heterocycles; we have also included several patents approved for the anticancer activity of piperazine heterocycles.

Similar content being viewed by others
References
P. Meena, V. Nemaysh, M. Khatri, et al., Bioorg. Med. Chem., 23(5), 1135 – 1148 (2015).
R. V. Patel, B. Mistry, R. Syed, et al., Eur. J. Pharm. Sci., 88, 166 – 177 (2016).
K. H. Rohde, H. A. Michaels, A. Nefzi, et al., Bioorg. Med. Chem. Lett., 9, 2206 – 2209 (2016).
M. Taha, M. Irshad, S. Imran, et al., Eur. J. Med. Chem., 141, 530 – 537 (2017).
M. Aggarwal, R. Kaur, A. Saha, et al., Antivir. Res., 146, 102 – 111 (2017).
M. Çeçen, J. M. Oh, Z. Özdemir, et al., Molecules, 22, 5371 (2020).
Y. Xu, P. Liang, H. Ur Rashid, et al., Med. Chem. Res., 10, 1618 – 1627 (2019).
B. N. Saðlýk, O. Cebeci, U. Acar Çevik, et al., Molecules, 18, 4342 – 4347 (2020).
G. Y. Zhang, Z. Zhang, K. Li, et al., Bioorg. Chem., 105, 104398 (2020).
Y. Chu, B. R. S. Reddy, V. P. R. Gajulapalli, et al., Bioorg. Med. Chem. Let., 24, 127613 (2020).
W. Li, S. Y. Chen, W. N. Hu, et al., J. Chem. Res., 9, 536 – 542 (2020).
K. S. Thriveni, B. Padmashali, M. B. Siddesh, et al., Ind. J. Pharm. Sci., 4, 332 – 338 (2014).
S. Radi, Y. Toubi, A. Hakkou, et al., Lett. Drug. Des. Discovery, 9, 853 – 857 (2012).
D. C. Batista, D. P. B. Silva, I. F. Florentino, et al., Inflammopharmacology, 26, 217 – 226 (2018).
E. E. Gurdal, E. Buclulgan, I. Durmaz, et al., Anticancer Agents Med. Chem., 15, 382 – 389 (2015).
N. Inceler, A. Yilmaz, S. N. Baytas, et al., Med. Chem. Res., 22, 3109 – 3118 (2013).
A. K. El-Damasy, S. H. Seo, N. C. Cho, et al., Eur. J. Med. Chem., 101, 754 – 768 (2013).
M. Al-Ghorbani, G. S. Pavankumar, P. Naveen, et al., Bioorg. Chem., 65, 110 – 117 (2016).
N. Perin, K. Bobanoviæ, I. Zlatar, Eur. J. Med. Chem., 125, 722 – 735 (2017).
H. H. Lin, W. Y. Wu, S. L. Cao, et al., Bioorg. Med. Chem. Lett., 23 3304 – 3307 (2013).
H. Chen, B. B. Xu, T. Sun, et al., Molecules, 22, 1857 – 1866 (2017).
M. K. Akkoç, M. Y. YükseL, Ý. Durmaz, et al., Turk. J. Chem., 4, 515 – 525 (2012).
M. S. R. Murty, M. R. Katiki, B. R. Rao, et al., Lett. Drug Des. Discovery, 9, 968 – 981 (2016).
J. R. Jiang, F. Xu, H. G. Wu, et al., J. Chem., 1, 2016 (2016).
L. Boddu, A. K. Pagudala, D. Gandamalla, et al., J. Mol. Struct., 1166, 362 – 368 (2018).
Z. Mao, X. Zheng, Y. Lina, et al., Bioorg. Med. Chem. Lett., 15, 3421 – 3424 (2016).
M. Tuncbilek, E. B. Guven, T. Onder, et al., J. Med. Chem., 55, 3058 – 3065 (2012).
C. Fytas, G. Zoidis, A. Tsotinis, et al., Eur. J. Med. Chem., 26, 281 – 290 (2015).
M. S. Murty, B. Ramalingeswara Rao, K. R. Ram, et al., Med. Chem. Res., 21, 3161 – 3169 (2012).
S. L. Cao, Y. Han, C. Z. Yuan, et al., Eur. J. Med. Chem., 64, 401 – 409 (2013).
R. Venkatesh, S. Kasaboina, D. Bidayat, et al., Bioorg. Med. Chem. Lett., 27, 354 – 359 (2017).
L. Yurttaş, S. Demirayak, S. Ilgýn, et al., Bioorg. Med. Chem., 22, 6313 – 6323 (2014).
M. Bu, T. Cao, H. Li, et al., Chem. Med. Chem., 6, 466 – 474 (2017).
H. M. Shallal, W. A. Russu, et al., Eur. J. Med. Chem., 46, 2043 – 2057 (2011).
Y. Zhang, Y. Huang, H. Xiang, et al., Eur. J. Med. Chem., 78, 23 – 34 (2014).
P. F. Geng, C. C. Wang, Z. H. Li, et al., Eur. J. Med. Chem., 143, 1959 – 1967 (2018).
S. B. Ozdemir, Y. U. Cebeci, H. Bayrak, et al., Heterocycl. Commun., 23, 43 – 54 (2017).
W. X. Sun, Y. J. Ji, Y.Wan, et al., Bioorg. Med. Chem. Lett., 27, 4066 – 4074 (2017).
Z. H. Li, T. Q. Zhao, X. Q. Liu, et al., Eur. J. Med. Chem., 1396 – 1405 (2018).
P. Wang, J. Huang, K. Wang, et al., Eur. J. Med. Chem., 122, 546 – 556 (2016).
Y. Zhang, C. R. Yang, X. Tang, et al., Bioorg. Med. Chem. Let., 19, 4666 – 4670 (2016).
R. Aeluri, M. Alla, S. Polepalli, N. Jain, et al., Eur. J. Med. Chem., 100, 18 – 23 (2015).
K. S. Kumar, A. Hanumappa, M. Hegde, et al., Eur. J. Med. Chem., 81, 341 – 349 (2014).
H. N. Nagesh, N. Suresh, G. V. Prakash, et al., Med. Chem. Res., 24, 523 – 532 (2015).
J. Sun, S. Z. Ren, X. Y. Lu, et al., Bioorg. Med. Chem., 25, 2593 – 2600 (2017).
L. K. Filak, D. S. Kalinowski, T. J. Bauer, et al., Inorg. Chem., 53, 6934 – 6943 (2014).
R. V. Patel, P. K. Patel, P. Kumari, et al., Eur. J. Med. Chem., 53, 41 – 51 (2011).
L. Nagarapu, H. K. Gaikwad, R. Bantu, et al., Eur. J. Med. Chem., 46, 2152 – 2156 (2011).
S. Kumar, N. Kumar, P. Roy, et al., Med. Chem. Res, 22, 4600 – 4609 (2013).
M. N. Aboul-Enein, A. M. El-Azzouny, F. A. Ragab, et al., Arch. Pharm. Chem. Life Sci., 3, 350 (2017).
C. B. Mishra, R. K. Mongre, S. Kumari, et al., ACS Chem. Biol.,12, 753 – 768 (2017).
M. El-Miligy, H. A. Abd El Razik, M. M. Abu-Serie, et al., Future Med. Chem., 9, 1709 – 1729 (2017).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Ghorbani, M., Gouda, M.A., Baashen, M. et al. Piperazine Heterocycles as Potential Anticancer Agents: A Review. Pharm Chem J 56, 29–37 (2022). https://doi.org/10.1007/s11094-022-02597-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02597-z