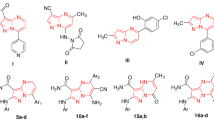
Bacterial drug resistance has become a growing problem worldwide due to the excessive use of antibiotics in recent decades. Two small focused libraries of 5H-pyrimido[5,4-b]indole-4-carboxamides and 5H-pyrimido-[5,4-b]indole-4-ketones were designed as eudistomin Y3 and 1-acetyl-β-carboline (1-ABC) analogs and prepared via application of Inverse Electron-Demand Diels-Alder (IEDDA) reaction of 1,3,5-triazines and 3-aminoindoles. Compounds 2a and 2b were discovered to have activity against Mycobacterium bovis BCG with Minimum Inhibitory Concentration (MICs) values of 25 and 50 μg/mL respectively while compound 2e was against all three strains of Candida albicans tested with MIC values of 50 μg/mL. Moreover, compound 2e demonstrated synergistic antibacterial activity with fluconazol, which suggested that future drug candidates from this class of compounds could be used in combination with existing drugs to treat C. albicans infections.




Similar content being viewed by others
References
J. Davies and D. Davies, Microbiol. Mol. Biol. Rev., 74, 417 – 433 (2010).
E. D. Brown and G. D. Wright, Nature, 529, 336 – 343 (2016).
M. Mhondoro, N. Ndlovu, D. Bangure, et al., BMC Infect. Dis., 19, 1 – 9 (2019).
E. Christaki,, M. Marcou, and A. Tofarides, J. Mol. Evol., 88, 26 – 40 (2020).
B. M. Kyaw, S. Arora, K. Nwe Win, et al., Afr. J. Microbiol. Res., 5, 3684 – 3692 (2011).
N. Kaur, R. Prasad, and A. Varma, Int. J. Pharm. Biol. Sci., 4, 534 – 540 (2013).
S. B. Levy and B. Marshall, Nat. Med., 10 (12 Suppl.), S122-S129 (2004).
J. Herrmann, T. Lukezic, A. Kling, et al., Curr. Top. Microbiol. Immunol., 398, 339 – 363 (2016).
L. N. Silva, K. R. Zimmer, A. J. Macedo, et al., Chem. Rev., 116, 9162 – 9236 (2016).
P. Ashok, S. Ganguly, and S. Murugesan, Mini-Rev. Med. Chem., 13, 1778 – 1791 (2013).
F. A. Khan, A. Maalik, Z. Iqbal, et al., Eur. J. Pharmacol., 721, 391 – 394 (2013).
H. D. H. Showalter, J. Nat. Prod., 76, 455 – 467 (2013).
P. Ashok, S. Ganguly, and S. Murugesan, Drug Discov. Today, 19, 1781 – 1791 (2014).
A. E. Laine, C. Lood, and A. M. P. Koskinen, Molecules, 19, 1544 – 1567 (2014).
C. S. Lood and A. M. P. Koskinen, Chem. Heterocycl. Compd., 50, 1367 – 1387 (2015).
M. Zhang and D. Sun, Anti-Cancer Agents Med. Chem., 15, 537 – 547 (2015).
R. Cao, W. Peng, Z. Wang, et al., Curr. Med. Chem., 14, 479 – 500 (2007).
T. H. Won, J.-E. Jeon, S.-H. Lee, et al., Bioorg. Med. Chem., 20, 4082 – 4087 (2012).
B. S. Joshi, V. N. Kamat, and D. H. Gawad, Heterocycles, 7, 193 – 200 (1977).
H. J. Shin, H.-S. Lee, and D.-S. Lee, J. Microbiol. Biotechnol., 20, 501 – 505 (2010).
M. J. Balunas and A. D. Kinghorn, Life Sci., 78, 431 – 441 (2005).
B. Haefner, Drug Discov. Today, 8, 536 – 544 (2003).
P. Vuorela, M. Leinonen, P. Saikku, et al., Curr. Med. Chem., 11, 1375 – 1389 (2004).
G. Xu, L. Zheng, Q. Dang, et al., Synthesis, 45, 743 – 752 (2013).
F. Song, X. Liu, H. Guo, et al., Org. Lett., 14, 4770 – 4773 (2012).
Acknowledgments
This work was supported by the Fundamental Research Funds of Jilin University (No. 45006050152), the Sci-Tech Development Projects of Jilin Province in China (No. 20140309010YY and No. 20180414075GH, and Changchun Discovery Sciences, Ltd.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, G., Wei, Q., Song, F. et al. Design and Synthesis of Aza-β-Carboline Analogs and their Antibacterial Evaluation. Pharm Chem J 55, 365–372 (2021). https://doi.org/10.1007/s11094-021-02429-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02429-6