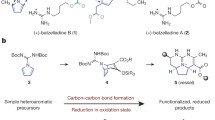
Harmicine, a chiral tetrahydro-β-carboline with a rare tetracyclic pyrrolidine framework, was isolated from the plant Kopsia griffithii in 1998. Before that, harmicine had already appeared frequently in the chemical literature as a starting material for natural product synthesis and it had been used as a model substrate in various methodology studies. Herein we review the relevant information available on this heterocyclic natural product before and after its isolation and classification as a natural product.



Similar content being viewed by others
References
I. Chakraborty and S. Jana, Synthesis, 45, 3325 (2013).
T.-S. Kam and K.-H. Lim, in: G. A. Cordell (editor ), The Alkaloids, Vol. 66, Academic Press, London (2008), p. 1.
3. R. B. Woodward, F. E. Bader, H. Bickel, A. J. Frey, and R. W. Kierstead, J. Am. Chem. Soc., 78, 2023 (1956).
M. Lounasmaa, P. Hanhinen, and M. Westersund, in: G. A. Cordell (editor), The Alkaloids, Vol. 52, Academic Press, San Diego (1999), p. 103.
M. Lounasmaa and A. Tolvanen, in: G. A. Cordell (editor), The Alkaloids, Vol. 42, Academic Press, San Diego (1992), p. 1.
T.-S. Kam and K.-M. Sim, Phytochemistry, 47, 145 (1998).
N. V. Koninklijke, Pharmaceutische Fabrieken v/h Brocades-Stheeman & Pharmacia, Pat. Appl. BE638408; Chem. Abstr., 62, 11817 (1965).
D. Din Belle, R. Jokela, A. Tolvanen, A. Haapalinna, A. Karjalainen, and J. Sallinen, WO Pat. Appl. 03082866.
H. M. Spindola, D. B. Vendramini-Costa, M. T. Rodrigues, Jr., M. A. Foglio, R. A. Pilli, and J. E. Carvalho, Pharmacol., Biochem. Behav., 102, 133 (2012).
S.-H. Qi, L. Miao, C.-H. Gao, Y. Xu, S. Zhang, and P.-Y. Qian, Helv. Chim. Acta, 93, 511 (2010).
C.-E. Nge, C.-Y. Gan, Y.-Y. Low, N. F. Thomas, and T.-S. Kam, Org. Lett., 15, 4774 (2013).
J. Kobayashi, M. Sekiguchi, S. Shimamoto, H. Shigemori, H. Ishiyama, and A. Ohsaki, J. Org. Chem., 67, 6449 (2002).
H. Ishiyama, M. Matsumoto, M. Sekiguchi, H. Shigemori, A. Ohsaki, and J. Kobayashi, Heterocycles, 66, 651 (2005).
T. H. Layne, S. McLean, W. F. Reynolds, and W. F. Tinto, Nat. Prod. Commun., 2, 649 (2007).
L. Zhang, C.-J. Zhang, D.-B. Zhang, J. Wen, X.-W. Zhao, Y. Li, and K. Gao, Tetrahedron Lett., 55, 1815 (2014).
K. Ahmad, Y. Hirasawa, A. E. Nugroho, A. H. A. Hadi, and H. Morita, Heterocycles, 86, 1611 (2012).
H. Irikawa, Y. Toyoda, H. Kumagai, and Y. Okumura, Bull. Chem. Soc. Jpn., 62, 880 (1989).
S. Yahara, H. Domoto, C. Sugimura, T. Nohara, Y. Niiho, Y. Nakajima, and H. Ito, Phytochemistry, 37, 1755 (1994)
G. Hahn and H. Werner, Ber. Dtsch. Chem. Ges., 71, 2163 (1938).
V. T. Wieland and E. Neeb, Liebigs Ann. Chem., 600, 161 (1956).
S. Corsano and S. Algieri, Ann. Chim. (Rome, Italy), 50, 75 (1960).
J. Harley-Mason, Pure Appl. Chem., 41, 167 (1975).
K. Nagarajan, C. Weismann, H. Schmid, and P. Karrer, Helv. Chim. Acta, 46, 1212 (1963).
G. Stork and R. K. Hill, J. Am. Chem. Soc., 79, 495 (1957).
E. Wenkert, S. Garratt, and K. G. Dave, Can. J. Chem., 42, 489 (1964).
J. P. Kutney, N. Abdurahman, P. Le Quesne, E. Piers, and I. Vlattas, J. Am. Chem. Soc., 88, 3656 (1966).
D. Herbst, R. Rees, G. A. Hughes, and H. Smith, J. Med. Chem., 9, 864 (1966)
D. R. Herbst and H. Smith, US Pat. Appl. 3943148.
G. H. Foster, J. Harley-Mason, and W. R. Waterfield, Chem. Commun. (London), 21a (1967).
B. A. Dadson, J. Harley-Mason, and G. H. Foster, Chem. Commun. (London), 1233a (1968).
B. A. Dadson and J. Harley-Mason, J. Chem. Soc., Chem. Commun. D, 665a (1969).
B. A. Dadson and J. Harley-Mason, J. Chem. Soc., Chem. Commun. D, 665b (1969).
J. Harley-Mason and C. G. Taylor, J. Chem. Soc., Chem. Commun. D, 812 (1970).
G. C. Crawley and J. Harley-Mason, J. Chem. Soc., Chem. Commun. D, 685 (1971).
M. J. Calverley, J. Chem. Soc., Chem. Commun., 1209 (1981).
W. R. Ashcroft, S. J. Martinez, and J. A. Joule, Tetrahedron, 37, 3005 (1981).
A. I. Meyers and S. Hellring, J. Org. Chem., 47, 2229 (1982).
A. I. Meyers, D. B. Miller, and F. H. White, J. Am. Chem. Soc., 110, 4778 (1988).
A. I. Meyers,T. Sohda, and M. Loewe, J. Org. Chem., 51, 3108 (1986).
A. I. Meyers and M. F. Loewe, Tetrahedron Lett., 25, 2641 (1984).
G. Schill, H. Löwer, C. U. Priester, U. F. Windhövel, and H. Fritz, Tetrahedron, 43, 3729 (1987).
G. Schill, C. U. Priester, U. F. Windhövel, and H. Fritz, Tetrahedron, 43, 3747 (1987).
G. Schill, C. U. Priester, U. F. Windhövel, and H. Fritz, Tetrahedron, 43, 3765 (1987).
S. B. Mandal, V. S. Giri, M. S. Sabeena, and S. C. Pakrashi, J. Org. Chem., 53, 4236 (1988).
R. C. Bernotas and R. V. Cube, Tetrahedron Lett., 32, 161 (1991).
J.-F. Carniaux, C. Kan-Fan, J. Royer, and H.-P. Husson, Tetrahedron Lett., 38, 2997 (1997).
B. Witkop, J. B. Patrick, and M. Rosenblum, J. Am. Chem. Soc., 73, 2641 (1951).
E. Winterfeldt, Liebigs Ann. Chem., 745, 23 (1971).
M. Nakagana, K. Matsuki, K. Hasegawa, and T. Hino, J. Chem. Soc., Chem. Commun., 742 (1982).
T. Itoh, M. Miyazaki, K. Nagata, M. Yokoya, S. Nakamura, and A. Ohsawa, Heterocycles, 58, 115 (2002).
T. Itoh, M. Miyazaki, K. Nagata, S. Nakamura, and A. Ohsawa, Heterocycles, 63, 655 (2004).
E. Wenkert and D. P. Roychaudhuri, J. Am. Chem. Soc., 78, 6417 (1956).
F. Bohlmann, Angew. Chem., 69, 641 (1957).
T. Itoh, Y. Matsuya, Y. Enomoto, K. Nagata, M. Miyazaki, and A. Ohsawa, Synlett, 1799 (1999).
S.-H. Lim, K.-M. Sim, Z. Abdullah, O. Hiraku, M. Hayashi, K. Komiyama, and T.-S. Kam, J. Nat. Prod., 70, 1380 (2007).
H.-J. Knölker and S. Agerwal, Synlett, 1767 (2004).
S. Agerwal and H.-J. Knölker, Org. Biomol. Chem., 2, 3060 (2004).
T. Kawate, M. Nakagawa, H. Yamazaki, M. Hirayama, and T. Hino, Chem. Pharm. Bull., 41, 287 (1993).
Review on the Bischler–Napieralski reaction: W. M. Whaley and T. R. Govindachari, in: R. Adams (editor), Organic Reactions, Vol. VI, John Wiley, New York (1951), p. 74.
Mechanistic investigation of the Bischler–Napieralski reaction: G. Fodor and S. Nagubandi, Tetrahedron, 36, 1279 (1980).
Mechanistic considerations regarding the Bischler–Napieralski reaction of N-acyltryptamines: J. R. Frost, B. R. P. Gaudillière, and A. E. Wick, J. Chem. Soc., Chem Commun., 895 (1985).
B. Hoefgen, M. Decker, P. Mohr, A. M. Schramm, S. A. F. Rostom, H. El-Subbagh, P. M. Schweikert, D. R. Rudolf, M. U. Kassack, and J. Lehmann, J. Med. Chem., 49, 760 (2006).
N. Uematsu, A. Fujii, S. Hashiguchi, T. Ikariya, and R. Noyori, J. Am. Chem. Soc., 118, 4916 (1996).
J. Szawkało, S. J. Czarnocki, A. Zawadzka, K. Wojtasiewicz, A. Leniewski, J. K. Maurin, Z. Czarnocki, and J. Drabowicz, Tetrahedron: Asymmetry, 18, 406 (2007).
L. Evanno, J. Ormala and P. M. Pihko, Chem.-Eur. J., 15, 12963 (2009).
F. Wang, H. Liu, L. Cun, J. Zhu, J. Deng, and Y. Jiang, J. Org. Chem., 70, 9424 (2005).
W. A. da Silva, M. T. Rodriguez, N. Shankaraiah, R. B. Ferreira, C. K. Z. Andrade, R. A. Pilli, and L. S. Santos, Org. Lett., 11, 3238 (2009).
A. González-Gómez, G. Domínguez, and J. Pérez-Castells, Tetrahedron, 65, 3378 (2009).
S. Saha, C. V. R. Reddy, and B. Patro, Tetrahedon Lett., 52, 4014 (2011).
R. V. Stevens, Acc. Chem. Res., 10, 193 (1977).
S. Mangalaraj and C. R. Ramanathan, RSC Adv., 2, 12665 (2012).
Review on the Pictet–Spengler reaction: E. D. Cox and J. M. Cook, Chem. Rev., 95, 1797 (1995).
Review on the Pictet–Spengler reaction: J. Stöckigt, A. P. Antonchick, F. Wu, and H. Waldmann, Angew. Chem., Int. Ed., 50, 8538 (2011).
S. M. Allin, S. N. Gaskell, M. R. J. Elsegood, and W. P. Martin, Tetrahedron Lett., 48, 5669 (2007).
S. M. Allin, C. I. Thomas, J. E. Allard, M. Duncaton, M. R. J. Elsegood, and M. Edgar, Tetrahedron Lett., 44, 2335 (2003).
F. D. King, J. Heterocycl. Chem., 44, 1459 (2007).
D. Ghislieri, A. P. Grenn, M. Pontini, S. C. Willies, I. Rowles, A. Frank, G. Grogan, and N. J. Turner, J. Am. Chem. Soc., 135, 10863 (2013).
G. Cami-Kobeci, P. A. Slatford, M. K. Whittlesey, and J. M. J. Williams, Bioorg. Med. Chem. Lett., 15, 535 (2005).
M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 126, 10558 (2004).
I. T. Raheem, P. S. Thiara, E. A. Peterson, and E. N. Jacobsen, J. Am. Chem. Soc., 129, 13404 (2007).
W.-H. Chiou, G.-H. Lin, C.-C. Hsu, S. J. Chaterpaul, and I. Ojima, Org. Lett., 11, 2659 (2009).
C. Sanaboina, S. Jana, S. Chidara, B. Patro, G. B. Raolji, and L. Eppakayala, Tetrahedron Lett., 53, 5027 (2012).
J. Seayad, A. M. Seayad, and B. List, J. Am. Chem. Soc., 128, 1086 (2006).
D. Huang, F. Xu, X. Lin, and Y. Wang, Chem.-Eur. J., 18, 3148 (2012).
E. J. Karppanen and A. M. P. Koskinen, Molecules, 15, 6512 (2010).
B. D. Christie and H. Rapoport, J. Org. Chem., 50, 1239 (1985).
O. K. Karjalainen and A. M. P. Koskinen, Org. Biomol. Chem., 10, 4311 (2012).
C. S. Lood and A. M. P. Koskinen, Eur. J. Org. Chem., 2357 (2014).
C. Dong, F. Mo, and J. Wang, J. Org. Chem., 73, 1971 (2008).
F. Mo, F. Li, D. Qui, Y. Zhang, and J. Wang, Chin. J. Chem., 30, 2297 (2012).
D. Fokas and J. A. Hamzik, Synlett, 581 (2009).
D. Fokas, M. Kaselj, Y. Isome, and Z. Wang, ACS Comb. Sci., 15, 49 (2013).
A. Akdemir, P. Rucktooa, A. Jongejan, R. van Elk, S. Bertrand, T. K. Sixma, D. Bertrand, A. B. Smit, R. Leurs, C. de Graaf, and I. J. P. de Esch, Bioorg. Med. Chem., 19, 6107 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Professor Gunars Duburs on the occasion of his 80th birthday.
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1488-1509, October, 2014.
Rights and permissions
About this article
Cite this article
Lood, C.S., Koskinen, A.M.P. Harmicine, a Tetracyclic Tetrahydro-β-Carboline: From the First Synthetic Precedent to Isolation from Natural Sources to Target-Oriented Synthesis (Review)* . Chem Heterocycl Comp 50, 1367–1387 (2015). https://doi.org/10.1007/s10593-014-1602-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1602-4