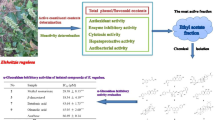
Various parts of Crataegus oxyacantha (hawthorn) plant have been used in traditional medicine in many countries including Algeria. In this study, antioxidant (GOR, ABTS, FRAP, CUPRAC and phenanthroline), photoprotective, and inhibition ability of n-butanol and ethyl acetate extracts from leaves and fruits of C. oxyacantha against Alzheimer’s disease [acetylcholinesterases (AChE) and butyrylcholinesterases (BChe)] and diabetes mellitus (α-glucosidase) related enzyme activities were investigated using standard methods. According to the obtained results, all plant extracts produced significant antioxidant effects. The ethyl acetate extract of C. oxyacantha leaves having the highest total bioactive content exhibited most pronounced antioxidant potential. Moreover, the extracts exhibited inhibitory effect against α-glucosidase, which was concentration dependent. The most potent inhibitor for α-glucosidase was n-butanol extract of C. oxyacantha leaves (with IC50 = 4.99 ± 0.82 μg/mL). The results also indicated a substantial BChe inhibitory activity of n-butanol extract (IC50 = 175.35 ± 16.25 μg/mL) and ethyl acetate extract (IC50 = 148.23 ± 13.41 μg/mL). The n-butanol extract was also the most potent inhibitor of AChE (IC50 = 159.09 ± 7.68 μg/mL. In addition, the plant extracts exhibited significant photoprotective potential with sun protection factor (SPF) values ranging from 17.19 ± 0.10 to 48.66 ± 0.00. Therefore, C. oxyacantha plant has a significant potential for enhancing pharmaceutical formulations.
Similar content being viewed by others
References
Y. J. Zhang, R. Y. Gan, S. Li, et al., Molecules, 20, 21138 – 56 (2015).
I. E. Orhan, Curr. Med. Chem., 25, 4854 – 4865 (2018).
M. Saoudi, R. B. Slamα-Ben Salem, M. B. Salem, et al., Biomed. Pharmacother., 114, 108795 (2019).
A. Mecheri, A. Amrani, W. Benabderrahmane, et al., Phytothérapie, 16S1, S22–S31 (2018).
A. Mecheri, W. Benabderrahmane, A. Amrani, et al., Recent Pat. Food Nutr. Agric., 10, 70 – 75 (2019).
W. Benabderrahmane, M. Lores, O. Benaissa, et al., Nat. Prod. Res., (2019); https://doi.org/10.1080/14786419.2019.1582044.
W. Benabderrahmane, M. Lores, J. P. Lamas, et al., Nat. Prod. Res., 32, 1220–1223 (2017).
M. Ali, S. Muhammad, M. R. Shah, et al., Front. Pharmacol., 8, 327 (2017).
T. Wresdiyati, S. Sa’diah, A. Winarto, et al., HAYATI J. Biosci., 22, 73 – 78 (2015).
A. J. Scheen. Drugs, 63, 933 – 51(2003).
H. Li, F. Song, J. Xing, et al., J. Am. Soc. Mass Spectrom., 20, 1496 – 503 (2009).
V. L. Singleton, J. A. J. Rossi, Amer. J. Enol. Viticult., 16, 144 – 58 (1965).
G. Topcu, A. Ay, A. Bilici, et al., Food Chem., 103, 816 – 822 (2007).
A. Kumaran and R. J. Karunakaran, LWT, 40, 344 – 352 (2007).
H. Shi, N. Noguchi, E. Niki, et al., Meth. Enzymol., 335, 157 – 66 (2001).
R. Re, N. Pellegrini, A. Proteggente, et al., Free Radical Biol. Med., 26, 1231–1237 (1999).
M. Ozturk, M. E. Duru, S. Kivrak, et al., Food Chem. Toxicol., 49, 1353 – 60 (2011).
A. Szydlowska-Czerniaka, C. Dianoczki, K. Recseg, et al., Talanta, 76, 899 – 905 (2008).
R. Apak, K. Guclu, M. Ozyurek, et al., J. Agric. Food Chem., 52, 7970 – 7981 (2004).
M. Oyaizu, Jpn. J. Nutr., 44, 307 – 315 (1986).
G. L. Ellman, D. Courtney, V. Andres, et al., Biochem. Pharmacol., 7, 88 – 95 (1961).
S. Lordan, T. J. Smyth, A. Soler-Vila, et al., Food Chem., 141, 2170 – 2176 (2013).
J. S. Mansur,M. N. R. Breder,M. C. A. Mansur, et al., An. Bras. Dermatol. Rio De Janeiro, 61, 121 – 124 (1986).
R. M. Sayre, P. P. Agin, G. J. Levee, et al., Photochem. Photobiol., 29, 559 – 566 (1979).
W. Mahmood, H. Saleem, W. Shahid, et al., Biocatal. Agric Biotechnol., 18, 101039 (2019).
D. Zheleva-Dimitrova. Pharmacogn Mag, 9, 109–113 (2013).
A. Ali Reza, M. S. Hossain, S. Akhter, et al., BMC Complement. Altern. Med., 18, 123 (2018).
H. Rasouli, S. M. Hosseini-Ghazvini, H. Adibi, et al., Food Funct., 8, 1942 – 1954 (2017).
H. Jouad, A. Lemhadri, M. Maghrani, et al., J. Herb. Pharmacother., 3, 19 – 29 (2003).
J. C. Mejia-Giraldo, K. Henao-Zuluaga, C. Gallardo, et al., Photochem. Photobiol., 92, 150 – 157 (2015).
A. Amrani, A. Mecheri, C. Bensouici, et al., Biocatal. Agric. Biotechnol., 20, 101209 (2019).
A. Jarzycka, A. Lewiñska, R. Gancarz, et al., J. Photochem. Photobiol. B, 128, 50 – 57 (2013).
R. G. De-Oliveira-Junior, C. A. A. Ferraz, G. R. Souza, et al., Biotechnol. Biotechnol. Equit., 31, 600 – 605 (2017).
R. Stevanato, M. Bertelle, and S. Fabris, Regul. Toxicol. Pharmacol., 69, 71 – 77 (2014).
Acknowledgments
The authors wish to express thanks to the Algerian Ministry of Higher Education and Scientific Research for PRFU Project (D01N01UN250120180009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mecheri, A., Amrani, A., Benabderrahmane, W. et al. In Vitro Pharmacological Screening of Antioxidant, Photoprotective, Cholinesterase, and α-Glucosidase Inhibitory Activities of Algerian Crataegus oxyacantha Fruits and Leaves Extracts. Pharm Chem J 54, 1150–1156 (2021). https://doi.org/10.1007/s11094-021-02334-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02334-y