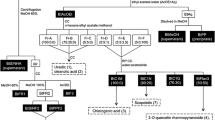
Abstract
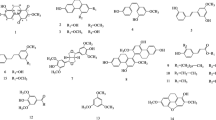
Elsholtzia rugulosa Hemsl., a species of the Labiatae family, has a long history of use as a honey plant, herbal tea, and folk medicine in China. However, little is known about its composition and biological activities. The present study aimed to investigate the total phenol and flavonoid contents, phytochemical composition, and multiple biological activities of this plant. The total flavonoid content of the ethyl acetate fraction (EAF) was higher than those of the petroleum ether fraction (PEF), n-butanol fraction (NBF), and water fraction (WF). The EAF also had much stronger antioxidant, cytotoxic, hepatoprotective, and acetylcholinesterase (AChE) and α-glucosidase inhibitory activities than the PEF, NBF, and WF. More importantly, the IC50 values of the EAF and NBF against α-glucosidase were much lower than that of the positive control acarbose, indicating their potent α-glucosidase inhibitory activities. The isolation of the EAF led to the acquisition of 9 compounds, four of which (β-daucosterol, methyl rosmarinate, betulinic acid, and oleanolic acid) possessed significant α-glucosidase inhibitory activities. Maltol 6’-O-(5-O-p-coumaroyl)-β-D-apiofuranosyl-β-D-glucopyranoside and rosmarinic acid were the major phenolic compounds in the EAF according to the HPLC-DAD analysis. All these findings indicate that the EAF, NBF, and some isolated compounds have the potential to be developed as antidiabetic drugs. Moreover, the dual inhibition of AChE and butyrylcholinesterase (BChE) of certain fractions indicates their potential in the development of anti-Alzheimer’s disease drugs. The present study provides a new understanding of the phytochemistry and bioactivity of E. rugulosa.
Graphical Abstract

Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Editorial Committee of the Flora of China of Chinese Academy of Science (1977) Flora of China (Vol 66). Science Press, Beijing, p 304, 308
Guo Z, Liu Z, Wang X, Liu W, Jiang R, Cheng R, She G (2012) Elsholtzia: phytochemistry and biological activities. Chem Cent J 6:147. https://doi.org/10.1186/1752-153X-6-147
Zhong JD, Zhao XW, Chen XQ, Li HM, Chen CH, Xia XS, Li RT (2016) Two new ursane-type triterpenoid saponins from Elsholtzia bodinieri. Arch Pharm Res 39:771–777. https://doi.org/10.1007/s12272-016-0750-8
Zhong JD, Zhao XW, Li HM, Gao LH, Li RT (2016) Five new oleanane triterpenoid saponins from the aerial parts of Elsholtzia bodinieri. Helv Chim Acta 99:204–209. https://doi.org/10.1002/hlca.201500195
Xiang L, Zhang L, Chen X, Xia X, Li R, Zhong J (2019) Ursane-type triterpenoid saponins from Elsholtzia bodinieri. Nat Prod Res 33:1349–1356. https://doi.org/10.1080/14786419.2018.1477144
Nugroho A, Park JH, Choi JS, Park KS, Hong JP, Park HJ (2019) Structure determination and quantification of a new flavone glycoside with anti-acetylcholinesterase activity from the herbs of Elsholtzia ciliata. Nat Prod Res 33:814–821. https://doi.org/10.1080/14786419.2017.1413556
Pudziuvelyte L, Liaudanskas M, Jekabsone A, Sadauskiene I, Bernatoniene J (2020) Elsholtzia ciliata (Thunb.) Hyl. extracts from different plant parts: Phenolic composition, antioxidant, and anti-inflammatory activities. Molecules 25(5):1153. https://doi.org/10.3390/molecules25051153
Pudziuvelyte L, Stankevicius M, Maruska A, Petrikaite V, Ragazinskiene O, Draksiene G, Bernatoniene J (2017) Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind Crop Prod 107(15):90–96. https://doi.org/10.1016/j.indcrop.2017.05.040
Seo YH, Trinh TA, Ryu SM, Kim HS, Choi G, Moon BC, Shim SH, Jang DS, Lee D, Kang KS, Lee J (2020) Chemical constituents from the aerial parts of Elsholtzia ciliata and their protective activities on glutamate-induced HT22 cell death. J Nat Prod 83(10):3149–3155. https://doi.org/10.1021/acs.jnatprod.0c00756
Nguyen DTX, Tran H, Schwaiger S, Stuppner H, Marzocco S (2021) Effect of non-volatile constituents of Elsholtzia ciliata (Thunb.) Hyl. From southern Vietnam on reactive oxygen species and nitric oxide release in macrophages. Chem Biodivers 18:e2000577. https://doi.org/10.1002/cbdv.202000577
Yang F, Pu HY, Yaseen A, Chen B, Li F, Gu YC, Shen XF, Wang MK, Guo DL, Wang L (2021) Terpenoid and phenolic derivatives from the aerial parts of Elsholtzia rugulosa and their anti-inflammatory activity. Phytochemistry 181:112543. https://doi.org/10.1016/j.phytochem.2020.112543
Zhao L, Hou L, Sun H, Yan X, Sun X, Li J, Bian Y, Chu Y, Liu Q (2011) Apigenin isolated from the medicinal plant Elsholtzia rugulosa prevents beta-amyloid 25-35-induces toxicity in rat cerebral microvascular endothelial cells. Molecules 16(5):4005–4019. https://doi.org/10.3390/molecules16054005
Zuo GY, Wang GC, Zhao YB, Xu GL, Hao XY, Han J, Zhao Q (2008) Screening of Chinese medicinal plants for inhibition against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). J Ethnopharmacol 120(2):287–290. https://doi.org/10.1016/j.jep.2008.08.021
Ma BX, Meng XS, Tong J, Ge LL, Zhou G, Wang YW (2018) Protective effects of Coptis chinensis inflorescence extract and linarin against carbon tetrachloride-induced damage in HepG2 cells through the MAPK/Keap1-Nrf2 pathway. Food Funct 9(4):2353–2361. https://doi.org/10.1039/C8FO00078F
Chen HQ, Zhang RR, Mei WL, Cai CH, Gai CJ, Yu XD, Dai HF (2019) A new eudesmane type sesquiterpene from cultivated Clerodendranthus spicatus in Hainan. China J Chin Mater Med 44(1):95–99. https://doi.org/10.19540/j.cnki.cjcmm.20181101.011
Woo ER, Piao MS (2004) Antioxidative constituents from Lycopus lucidus. Arch Pharm Res 27(2):173–176. https://doi.org/10.1007/BF02980102
Wei JF, Chang X, Wang W, Kang WY (2013) Chemical constituents from Lysimachia circaeoides. J Chin Med Mat 36(9):1441–1443. https://doi.org/10.13863/j.issn1001-4454.2013.09.024
Li H, Nakashima T, Tanaka T, Zhang YJ, Yang CR, Kouno I (2008) Two new maltol glycosides and cyanogenic glycosides from Elsholtzia rugulosa Hemsl. J Nat Med 62:75–78. https://doi.org/10.1007/s11418-007-0188-x
Huang H, Sun HD, Wang MS, Zhao SX (1996) Phenolic compounds of Isodon oresbius. J Nat Prod 59(11):1079–1080. https://doi.org/10.1021/np960430w
Lu XR, Liu S, Wang MY, Gong MX, Wang ZM, Chen XQ (2014) Triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia stems. China J Chin Mater Med 39(23):4629–4636. https://doi.org/10.4268/cjcmm20142329
Chi F, Deng J, Wang YH (2010) Chemical constituents from Lagotis brevituba. China J Chin Mater Med 35(7):869–871. https://doi.org/10.4268/cjcmm20100714
Njateng GSS, Du Z, Gatsing D, Donfack ARN, Talla MF, Wabo HK, Tane P, Mouokeu RS, Luo X, Kuiate JR (2015) Antifungal properties of a new terpernoid saponin and other compounds from the stem bark of Polyscias fulva Hiern (Araliaceae). BMC Complem Altern M 15:25. https://doi.org/10.1186/s12906-015-0541-7
Ismaili L, Refouvelet B, Benchekroun M, Brogi S, Brindisi M, Gemma S, Campiani G, Filipic S, Agbaba D, Esteban G, Unzeta M, Nikolic K, Butini S, Marco-Contelles J (2017) Multitarget compounds bearing tacrine- and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog Neurobiol 151:4–34. https://doi.org/10.1016/j.pneurobio.2015.12.003
Dra LA, Rodrigues MJ, Neng NR, Nogueira JMF, Elamine Y, Aghraz A, Markouk M, Larhsini M, Custódio L (2019) Exploring Caralluma europaea (Guss.) N.E.Br. as a potential source of bioactive molecules: In vitro antioxidant and antidiabetic properties, and phenolic profile of crude extracts and fractions. Ind Crop Prod 139:111527. https://doi.org/10.1016/j.indcrop.2019.111527
Kumar RV, Sinha VR (2012) Newer insights into the drug delivery approaches of α-glucosidase inhibitors. Expert Opin Drug Del 9(4):403–416. https://doi.org/10.1517/17425247.2012.663080
Salazar MO, Osella MI, Arcusin DEJ, Lescano LE, Furlan RLE (2020) New α-glucosidase inhibitors from a chemically engineered essential oil of Origanum vulgare L. Ind Crop Prod 156(15):112855. https://doi.org/10.1016/j.indcrop.2020.112855
Xue X, Guo Z, Zhang H, Liu X, Luo J, Li D, Li J (2016) Chemical composition, in vitro antioxidant activity and α-glucosidase inhibitory effects of the essential oil and methanolic extract of Elsholtzia densa Benth. Nat Prod Res 30(23):2707–2711. https://doi.org/10.1080/14786419.2015.1135147
Peng H, Xing Y, Gao L, Zhang L, Zhang G (2014) Simultaneous separation of apigenin, luteolin and rosmarinic acid from the aerial parts of the copper-tolerant plant Elsholtzia splendens. Environ Sci Pollut Res 21:8124–8132. https://doi.org/10.1007/s11356-014-2747-5
Zhong JD, Feng Y, Li HM, Xia XS, Li RT (2016) A new flavonoid glycoside from Elsholtzia bodinieri. Nat Prod Res 30(20):2278–2284. https://doi.org/10.1080/14786419.2016.1164698
Funding
This research was supported by the Qinglan Project of University of Jiangsu Province, the High-end Talents Supporting Project of Yangzhou University, and the Qinglan Project of Yangzhou University (20180210).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Additional information
The manuscript entitled with “Elsholtzia rugulosa: Phytochemical profile and antioxidant, anti-Alzheimer's disease, anti-diabetic, antibacterial, cytotoxic and hepatoprotective activities” by Liu et al is submitted to Plant Foods for Human Nutrition. The result in this manuscript has not been published or submitted elsewhere. All the authors have agreed the final version of the manuscript and our school agrees to the submission of this paper to the journal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1.69 MB)
Rights and permissions
About this article
Cite this article
Liu, L., Gao, Q., Zhang, Z. et al. Elsholtzia rugulosa: Phytochemical Profile and Antioxidant, Anti-Alzheimer’s Disease, Antidiabetic, Antibacterial, Cytotoxic and Hepatoprotective Activities. Plant Foods Hum Nutr 77, 62–67 (2022). https://doi.org/10.1007/s11130-021-00941-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-021-00941-4