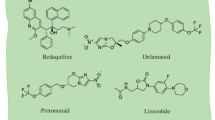
The historical classification (1993) of anti-tuberculosis drugs included three (I-III) lines that were subdivided into five groups (2006). The duration of treatment of drug-sensitive (DS) tuberculosis (TB) with first-line drugs is 6 – 12 months; of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB with second-line drugs, 18 months and longer. The Directly Observed Treatment Short course (DOTS) and DOTS-Plus therapies are measures of world TB control. Based on the need for more effective treatment of MDR and XDR TB, the classification of 2016 introduced six groups (A-C and D1-D3) in connection with the development of new anti-TB drugs. The present review presents first-line preparations and their new derivatives possessing activity against DS and drug-resistant (DR) M. tuberculosis strains.




Similar content being viewed by others
Notes
Efflux pump resistance mechanisms are responsible for transport of compounds such as neurotransmitters, toxic substances, and antibiotics from cells. This mechanism is important because it enhances MTB resistance to bacterial antibiotics. Efflux pump systems function by removing undesired toxic substances through special efflux pumps. Several efflux systems creating a barrier to drug penetration into the bacterial cell are specific for drugs while others can facilitate penetration of various drugs into bacterial cells, creating only slight multidrug resistance.
References
Treatment of TB: Guidelines for National Programmes. World Health Organization, WHO/TB/97.220, 1997.
World Health Organization. The treatment of tuberculosis guidelines. Document WHO/HTM/TB/2009.420, WHO, Geneva, 2010.
WHO consolidated guidelines on drug-resistant tuberculosis treatment, World Health Organization, Geneva, 2019; Licence: CC BY-NC-SA 3.0 IGO.
Fact Sheets Elimination Tuberculosis: General Information. October 2011; https://www.cdc.gov/tb/publications/factsheets/general/tb.htm.
R. Bayer and D. Wilkinson, Lancet, 345(8964), 1545 – 1548 (1995).
J. M. Grange, Int. J. Tuberc. Lung Dis., 1(4), 293 – 296 (1997).
World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis: www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/en/; Date last accessed: Dec. 12, 2012. Date last updated: 2011.
World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2006.361, World Health Organization, Geneva, 2006.
World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2014.11, World Health Organization, Geneva, 2014.
World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency update 2008. WHO/HTM/TB/2008.402, World Health Organization, Geneva, 2008.
The use of bedaquiline in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. World Health Organization, 2013.
World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis. Interim policy guidance, 2014.
A. Rendon, S. Tiberi, A. Scardigli, et al., J. Thorac. Dis., 8(10), 2666 – 2671 (2016).
E. Jaramillo, SESSION 5, Jun. 1, 2017. New and repurposed anti-TB drug introduction. WHO policies related with management of drug-resistant tuberculosis. Global TB Programme, WHO/HQ/LDR unit, Geneva, 2017.
P. Purkan, I. Ihsanawati, D. Natalia, et al., Ukr. Biochem. J., 88(5), 71 – 81 (2016).
Y.-Q. Hu, S. Zhang, F. Zhao, et al., Eur. J. Med. Chem., 133, 255 – 267 (2017).
R. Tandon and M. Nath, Mini-Rev. Med. Chem., 17(6), 549 – 570 (2017).
Z. Rychtarcikova, M. Kratky,M. Gazdova, et al., Molecules, 19, 3851 – 3868 (2014).
M. O. Rodrigues, J. B. Cantos, C. R. M. D’Oca, et al., Bioorg. Med. Chem., 21(22), 6910 – 6914 (2013).
D. K. Beena, G. Khare, S. Kidwai, et al., Eur. J. Med. Chem., 81, 301 – 313 (2014).
J. P. Raval, N. H. Patel, H. V. Patel, et al., Chem. Res., 20, 274 – 279 (2011).
G. F. dos S. Fernandes, P. S. de Souza, L. B. Marino, et al., Eur. J. Med. Chem., 123, 523 – 531 (2016).
G. F. dos S. Fernandes, P. C. de Souza, E. Moreno-Viguri, et al., J. Med. Chem., 60(20), 8647 – 8660 (2017).
H. S. N. Kumar, T. Paramasivam, F. Jumaat, et al., Med. Chem. Res., 23, 269 – 279 (2014).
P. Kumar, P. Rawat, P. Singh, et al., Int. J. Pharm. Sci. Res., 4(6), 2080 – 2093 (2013).
N. Anand, K. Upadhyaya, and R. P. Tripathi, Chem. Biol. Interface, 5(2), 84 – 127 (2015).
B. Prideaux, L. E. Via, M. D. Zimmerman, et al., Nat. Med., 21, 1223 – 1227 (2015).
W. Manosuthi, S. Wiboonchutikul, and S. Sungkanuparph, AIDS Res. Ther., 13, 22 (2016).
M. Asif, World J. Org. Chem., 1, 14 – 19 (2013).
A. N. Unissa, L. E. Hanna, and S. Swaminathan, Chem. Biol. Drug Des., 87, 537 – 550 (2016).
T. Rawal and S. Butani, Indian J. Pharm. Sci., 78(1), 8 – 16 (2016).
“Adis Insight: Rifalazil (ABI 1648; KRM 1648; PA 1648)”, Adis Insight, Springer International Publishing AG, 2016.
K. E. Dooley, C. D. Mitnick, M. A. DeGroote, et al., Clin. Infect. Dis., 55(4), 572 – 581 (2012).
K.-W. Jo, W. Ji, Y. Hong, et al., Respir. Med., 107, 292 – 297 (2013).
K. E. Dooley, P. Sayre, J. Borland, et al., J. Acquired Immune Defic. Syndr., 62(1), 21 – 27 (2013).
T. R. Sterling, N. A. Scott, J. M. Miro, et al., AIDS (London, U. K.), 30(10), 1607 – 1615 (2016).
A. Jindani, T. S. Harrison, A. J. Nunn, et al., N. Engl. J. Med., 371(17), 1599 – 1608 (2014).
Treatment of Tuberculosis, Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 UPDATE, World Health Organization, 2017.
1 Month of TB prophylaxis as effective as 9 months, Infectious Disease. Respiratory Infect. Dis. News, Healio, 2018.
M. Njire, Y. Tan, J. Mugweru, et al., Adv. Med. Sci., 61(1), 63 – 71 (2016).
S. Sarkar, A. Ganguly, and H. H. Sunwoo, Mycobact. Dis., 6, 209 (2016).
Y. S. Kwon, Chonnam Med. J., 53(2), 103 – 109 (2017).
Z. Ahmad, S. Tyagi, A. Minkowski, et al., Indian J. Med. Res., 136(5), 808 – 814 (2012).
J. Zitko, B. Servusova, P. Paterova, et al., Molecules, 18(12), 14807 – 14825 (2013).
O. Jandourek, M. Tauchman, P. Paterova, et al., Molecules, 22, 223 – 243 (2017).
S. Zhou, S. Yang, and G. Huang, J. Enzyme Inhib. Med. Chem., 32(1), 1183 – 1186 (2017).
N. D. Segretti, C. K. Simoes, M. F. Correa, et al., Tuberculosis, 99, 11e16 (2016).
M. F. Correa and J. P. S. Fernandes, Curr. Protein Pept. Sci., 17(3), 213 – 219 (2016).
G. B. Migliori and A. Zumla, in: Infectious Diseases, 4th Ed., J. Cohen, W. G. Powderly, and S. M. Opal (eds.), Elsevier, Amsterdam, 2017, pp. 1264 – 1276.e2.
K. Kumar and A. V. Narsaiah, Org. Commun., 7(1), 28 – 33 (2014).
M. Asif, Elixir. Farmacy, 53, 11774 – 11778 (2012).
H. Safi, S. Lingaraju, A. Amin, et al., Nat. Genet., 45, 1190 – 1197 (2013).
K. Schubert, B. Sieger, F. Meyer, et al., mBio, 8(1), e02213 – 16 (2017).
R. E. Lee, M. Protopopova, E. Crooks, et al., J. Comb. Chem., 5(2), 172 – 187 (2003).
M. N. Protopopova, in: Advances in Tuberculosis Medicinal Chemistry, M. Kuroso and W. Denny (eds.), Future Science Ltd. — Unitec House, London (2016), Chap. 7, pp. 104 – 118.
D. Machado, M. Girardini, M. Viveiros, and M. Pieroni, Front Microbiol., 9, 1367 (2018).
O. K. Onajole, P. Govender, and P. D. van Helden, Eur. J. Med. Chem., 45, 2075 – 2079 (2010).
S. E. Borisov, E. M. Bogorodskaya, G. V. Volchenkov, et al., Tuber. Lung Dis., 96(3), 6 – 18 (2018).
M. J. Boeree, N. Heinrich, R. Aarnoutse, et al., Lancet Infect. Dis., 17(1), 39 – 49 (2017).
B. P. Goldstein, J. Antibiot., 67(9), 625 – 630 (2014).
C. Vilcheze, Jr., and W. Jacobs, Microbiol. Spectrum, 2(4), MGM2 – 0014 – 2013 (2014).
Y. Zhang, W. Shi, W. Zhang, and D. Mitchison, Microbiol. Spectrum, 2(4), 1 – 12 (2014).
N. Dookie, S. Rambaran, N. Padayatchi, et al., J. Antimicrob. Chemother., 73, 1138 – 1151 (2018).
J. Bacon, L. J. Alderwick, J. A. Allnutt, et al., PLoS One, 9(2), e87329 (2014).
J. A. Caminero (ed.), Guidelines for Clinical and Operational Management of Drug-Resistant Tuberculosis, International Union Against Tuberculosis and Lung Disease, Paris, France, 2013.
D. Falzon, F. Mirzayev, F. Wares, et al., Eur. Respir. J., 45, 150 – 160 (2015).
D. C. Bay and R. J. Turner, Small Multidrug Resistance Efflux Pumps, Springer International Publishing, Switzerland, 2016, pp. 45 – 71.
J. Sun, Z. Deng, and A. Yan, Biochem. Biophys. Res. Commun., 453(2), 254 – 267 (2014).
Y. Xu, J. Wu, S. Liao, et al., Ann. Clin. Microbiol. Antimicrob., 16(1), 1 – 13 (2017).
World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization. WHO/HTM/TB/2014.11, Geneva, 2014.
D. Falzon, H. J. Schunemann, E. Harausz, et al., World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update, Eur. Respir. J., 49(3), 1602308 (2017).
T. H. Keller, A. Pichota, and Z. Yin, Curr. Opin. Chem. Biol., 10, 357 – 361 (2006).
G. Piccaro, G. Poce, M. Biava, et al., J. Antibiot., 68, 711 – 714 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kayukova, L.A., Berikova, E.A. Modern Anti-Tuberculosis Drugs and Their Classification. Part I: First-Line Drugs. Pharm Chem J 54, 555–563 (2020). https://doi.org/10.1007/s11094-020-02239-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02239-2