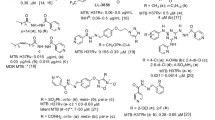
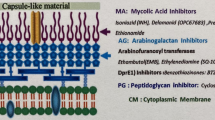
Abstract
Development of new drugs to treat drug-resistant tuberculosis is most important in the fight against the global spread of this contagious and potentially deadly infectious disease. Prior to 2010, no new class of drugs was introduced to treat tuberculosis (TB) for over 40 years. Although rifapentine (a long-acting rifamycin) was approved by the United States (US) Food and Drug Administration (FDA) in 1998 for the treatment of pulmonary TB, it was rarely used and is not effective for multidrug-resistant (MDR) TB. In the past decade, however, three new drugs (bedaquiline, delamanid, and pretomanid) have been introduced on the global scene to combat MDR- and extensive drug-resistant (XDR) TB. Their chemistry, pharmacology/pharmacokinetics, in vitro activity, and clinical efficacy and side effects are reviewed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
World Health Organization. Global tuberculosis report 2019. Geneva: World health Organization; 2019. https://www.who.int/tb/publications/global=-report/en
Ramchandani SR, Gupta A, Bensen CA, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001–11.
Dorman SE, Nahid P, Kurbata EV, et al. Four—month rifapentine regimens with or without moxifloxcacin for tuberculosis. N Engl J Med. 2021;384:1705–18.
Khoshnood S, Goudarzi M, Taki E, et al. Bedaquiline: current status and future perspectives. J Global Antimicrob Resistance. 2021;25:48–59.
Matteelli A, Carvalho AC, Dooley KE, Kritski A. TMC207: the first compound of a new class of potent anti-tuberculous drugs. Future Microbiol. 2010;5:849–58.
Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterial tuberculosis. Science. 2005;307:223–7.
Chahine EB, Karaoui LR, Mansour H. Bedaquiline: a novel diarylquinoline for multidrug-resistant tuberculosis. Annn Pharmacother. 2014;48:107–15.
Andries K, Villellas C, Coeck N, et al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One. 2014;9:e102135.
Ghajavand H, Kamakoli MK, Khanipour S, et al. High prevalence of bedaquiline resistance in treatment-naïve tuberculosis patients and verapamil effectiveness. Antimicrob Agents Chemother. 2019;63:e02530–18.
Nguyen TVA, Anthony RM, Banuls AL, Nguyen TVA, Vu DH, Alffenaar JWC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66:1625–30.
Bloomberg GV, Keller PM, Stucki D, et al. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. N Engl J Med. 2015;373:1986–8.
Villellas C, Coeck N, Meehan CJ, et al. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemothetr. 2016;72:684–90.
Liu Y, Gao M, Du J, et al. Reduced susceptibility of Mycobacterium tuberculosis to bedaquiline during antituberculous treatment and its correlation with clinical outcomes in China. Clin Infect Dis. 2021;73:e3391–7.
van Heeswijk RPG, Dannemann B, Hoetelmans RMW. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother. 20–14; 69: 2310-8.
Upton CM, Steele C, Maartens G, Diacon AH, Wiesner L, Dooley KE. Pharmacokinetics of bedaquiline in cerebrospinal fluid [CSF] in patients with pulmonary tuberculosis [TB]. J Antimicrob Chemother. 2022;77:dka067. https://doi.org/10.1093/jaqc/dkac067.
Gaida R, Truter I, Peters CA. Adverse effects of bedaquiline in patients with extensively drug-resistant tuberculosis. S Afr J Infect Dis. 2020;35:23.
Olayanju O, Limberis J, Esmail A, et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51:18005444.
Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicenter study. Eur Respir J. 2017;49:1700387.
Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommended treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57:20003300.
Wang M-G, Wu S-Q, He J-Q. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: a systemic review and meta-analysis. BMC Infect Dis. 2021;21:970.
World health Organization. WHO best-practice statement on the off-label use of bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis. Geneva: World Health Organization; 2017. p. 2017.
WHO. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Available at:https://www.who.int/tb/publications/2019/consolidated-guiidelines-drug-resistant-TBtreatment/en/.Accessed 23 Nov 2021.
Matsumoto M, Hashizume H, Tomishinge T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3:e466.
Nguyen TVA, Anthony RM, Cao TTH, et al. Delamanid resistance: update and clinical management. Clin Infect Dis. 2020;71:3252–9.
Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid [OPC-67683] in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15:949–54.
Wen S, Jing W, Zhang T, et al. Comparison of in vitro activity of the nitroimidazoles delamanid and pretomanid against multi-drug resistant and extensive drug-resistant tuberculosis. Eur J Clin Microbial Infect Dis. 2019;38:1293–6.
Simson K, Kurepina N, Venter A, et al. MIC of delamanid [OPC-67683] against Mycobacterium tuberculosis clinical isolates and a proposed critical concentration. Antimicrob Agents Chemother. 2016;60:3316–22.
Pang Y, Zong Z, Huo F, et al. In vitro susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61:e00900–17.
Yang JS, Kim KJ, Choi H, Lee SH. Delamanid, bedaquiline, and linezolid minimum inhibitory concentration distributions and resistance related gene mutations in multidrug-resistant tuberculosis in Korea. Ann Lab Med. 2018;38:563–8.
Kempker RR, Mikiashvilli L, Zhao Y, et al. Clinical outcomes among patients with drug-resistant tuberculosis receiving bedaquiline- or delamanid-containing regimens. Clin Infect Dis. 2020;71:23336–44.
Rifat D, Li SY, Ioerger T, et al. Mutations in RV2983 as a novel determinant of resistance to nitroimidazole drugs in Mycobacterium tuberculosis. bioRxiv. 2018:457754. https://doi.org/10.1101/457754.
Wang X, Mallikaarjun S, Gibiansky E. Population pharmacokinetic analysis of delamanid in patients with pulmonary multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2021;65:e01202-20.
Gler MT, Skripconoka V, Sanchez-Garvito E, et al. Delamanid for multidrug-resistant tuberculosis. N Engl J Med. 2012;366:2151–60.
Stancil SL, Mirzayev F, Abdel-Rahman SM. Profiling pretomanid as a therapeutic option for TB infection: evidence to date. Dovepress. 2021;15:2815–30.
McGrath M, Gey van Pitius NC, van Helden PD, Warren RM, Warner DF. Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2014;69:292–302.
Kadura S, King N, Nakhoul M, Zhu H, Theron G, Koser C, Farhal M. Systemic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother. 2020;75:2031–43.
Lee BM, Almeida DV, Afriat-jurnou L, et al. The evolution of nitroimidazole antibiotic resistance in Mycobacterium tuberculosis. bioRxiv. 2019;63:631127.
Salinger DH, Subramoney V, Everitt D, Nedelman JR. Population pharmacokinetics of the antituberculous agent pretomanid. Antimicrob Agents Chemother. 2019;63:e00907–19.
Glis T, Lynen L, de Jung BC, Van Duen A, Decroo T. Pretomanid for tuberculosis: a systemic review. Clin Microbial Infect. 2021; https://doi.org/10.1016/j.c.mi.2021.08.007.
Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902.
Ndjeka N, Campbell JR, Meintjes G, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect Dis. 2022. Published online May 2, 2022;22:1042. https://doi.org/10.1016/S1473-3099[21]00811-2.
Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387:810–22.
World Health Organization. Rapid communication: key changes to the treatment to the treatment of drug-resistant tuberculosis. May 2, 2022. https://www.whoint/publications/i/item/WHO-UCN-TB-2022-2
Hewison C, Khan U, Bastard M, et al. Safety of treatment regimens containing bedaquiline and delamanid in the endTB cohort. Clin Infect Dis. 2022. Published online Jan 13;75:1006. https://doi.org/10.1093/cid/ciao19.
Huerga H, Khan U, Bastard M, et al. Safety and effectiveness outcomes from a 14-country cohort of patients with multi-drug resistant tuberculosis treated concomitantly with bedaquiline, delamanid and other second-line drugs. Clin Infect Dis. 2022:ciac176. https://doi.org/10.1093/cid/ciac176.
Yoshiyama T, Takaki A, Aono A, Mitarai S, Okumura M, Ohta K, Kato S. Multidrug resistant tuberculosis with simultaneous acquired drug resistance to bedaquiline and delamanid. Clin Infect Dis. 2021;73:2329–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fong, I.W. (2023). New Anti-tuberculous Drugs: Bedaquiline, Delamanid, and Pretomanid. In: New Antimicrobials: For the Present and the Future. Emerging Infectious Diseases of the 21st Century. Springer, Cham. https://doi.org/10.1007/978-3-031-26078-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-26078-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26077-3
Online ISBN: 978-3-031-26078-0
eBook Packages: MedicineMedicine (R0)