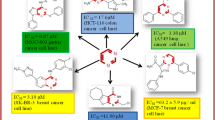
Through a structure-based molecular hybridization strategy, a series of new N-acylhydrazone derivatives containing the benzothiazole and indole based moiety were designed, synthesized and screened for in vitro antiproliferative activity against Hep G2 cancer cell line. One compound (7a) exhibited excellent antiproliferative activity with IC50 values of 0.78 μM against Hep G2. In addition, C-5 substitutions of the indole ring of target compounds might be crucial for their cytotoxic activities. Additionally, the relative configuration of target compounds was confirmed as the E isomer. Further chemical manipulation of derivative 7a can make it possible to obtain new potential antitumor agents.
Similar content being viewed by others
1. Introduction
Cancer is one of the most life-threatening diseases worldwide. Despite tremendous efforts to develop effective treatment strategies, satisfactory results have not yet been obtained, and the overall survival rate and disease-free survival rate of most cancers are still very low [1, 2]. Therefore, discovery of more effective and safer anticancer agents remains urgently needed.
Indole, as a typical N-heterocyclic aromatic compound is widespread in nature, It has been considered as an important structural component in many pharmaceutical agents including antidepressant [3], anticonvulsant [4], antifungal [5], antiviral [6] anti-inflammatory [7], and particularly in discovery of new antitumor agents [8, 9]. In recent years, more new indole derivatives have been developed as potent antitumor agents, including cediranib (1) [10], obatoclax (2) [11], D-24851 (3) and sunitinib (4) [12] (Fig. 1).
Benzothiazoles are heterocyclic compounds with diverse range of biological activities. Many studies reported that benzothiazoles derivatives exhibited various activities, including antimicrobial [13], anti-inflammatory [14], antifungal [15] anticancer [16], antidiabetic [17], anticonvulsant [18], antiviral [19], antitubercular [20], antimalarial [21], and antihelmintic [22]. Particularly, 2-substituted benzothiazoles are among privileged structures in medicinal chemistry [23, 24]. Recently, some 2-substituted benzothiazole derivatives have been evaluated as potential antitumor agents 5 [25] and 6 [26] (Fig. 2).
According to structure-based molecular hybridization strategy, researchers have attempted to combine benzothiazole and indole through different linkers to obtain new candidates with useful pharmacological activity [27, 28]. The N-acylhydrazone (NAH) scaffold is usually employed in the design of heterocyclic compounds, which endow molecules with good thermal, hydrolytic, and chemical stability, as well as the feasibility of changing chemical composition by various substituents [29, 30]. In recent years, some compounds containing NAH scaffold have been reported as antitumor agents, e.g., PAC-1 (7) [31], LASSBio-1586 (8) [32], and compound 9 [33] (Fig. 2), and it was indicated that introduction of NAH as a linker was an effective approach for developing more potent and broad spectrum antitumor agents.
In this study, based on a structure-based molecular hybridization strategy – a common and effective approach to developing small molecules with multi-targeted molecular mechanism, we have designed and synthesized a series of new NAH derivatives containing the benzothiazole and indole- based moiety (Fig. 3). Herein, twelve new NAH derivatives and their antiproliferative activities in vitro against Hep G2 cancer cell line are reported. Additionally, the configuration of target compounds was confirmed as the E isomer. The proposed structures of target compounds were confirmed by 1H NMR, 13C NMR, NOESY NMR, and IR spectroscopy data.
2. Experimental Chemical Part
2.1 Chemicals and Instruments
All chemicals and solvents were obtained from commercial sources and used without further purification. Reactions were monitored by thin layer chromatography (TLC) using glass plates precoated with a layer of silica gel (GF254) containing a fluorescent indicator. TLC plates were visualized by exposure to ultraviolet light (254 nm). The melting points of synthesized compounds were determined in an electric melting point apparatus and remained uncorrected. The 1H NMR and 13C NMR spectra were measured on 500 MHz Bruker spectrometer in DMSO-d6 using TMS as internal standard. Infrared (IR) spectra were recorded on Nicolet 370 FT-IR spectrometer.
2.2 Synthetic Routes
An efficient method for the synthesis of linked N-acylhydrazone derivatives containing the benzothiazole and indole-based moiety (7a – 7e, 8a – 8g) was developed as shown in Schemes 1 and 2. According to a convergent synthesis approach, we initially synthesized the key intermediates benzothiazole-2-carbohydrazide (3) and substituted 1H-indole-3-carbaldehydes (5a – 5e, 6a – 6g) to afford the proposed acylhydrazones. As illustrated in Scheme 1, condensation of compound 1 with diethyl oxalate furnished compound 2, which was reacted with hydrazine to obtain target hydrazides 3.
The synthetic route to compounds 4a – 4e is outlined in Scheme 2. Under Vilsmeier-Haack (DMF-POCl3) conditions, compounds 4a – 4e were transformed into the corresponding 3-carboxaldehyde functionalized indoles 5a – 5e. The N-ethyl, N-isopropyl and N-phenylsulfonyl derivatives (6a – 6g) of indole-3-carboxaldehyde were obtained via reaction of 5a – 5c with bromoethane, 2-bromopropane or benzenesulfonyl chloride in the presence of sodium hydroxide (NaOH) in DMSO and water. This was the first time that DMSO and water were used as a mixed solvent for this reaction and such a high yield has not been reported before. Finally, the condensation of compound 3 with compounds 5a – 5e or compounds 6a – 6g, produced the target compounds 7a – 7e and 8a – 8g. In summary, this synthetic route for a series of N-acylhydrazone derivatives containing the benzothiazole and indole-based moiety shows significant advantages, such as mild conditions, high yields, and simple operation process. The results are summarized in Table 1.
Due to the presence of an imino bond, all target compounds 7a – 7-e and 8a – 8g could exist in either E or Z isomeric form, hence, the next step in this work was to determine the relative configuration of the imino double bond in target compounds. By careful analysis of the 1H-NMR spectra of compounds 7a – 7e and 8a – 8g, we only detected the presence of one imino hydrogen signal (Table 1). According to the literature [34], this was attributed to the presence of only (E)-diastereomers in N-acylhydrazone derivatives. Compound 7a was further selected to identify the stereochemistry by undergoing NOESY NMR. Results (Fig. 4) showed that an evident NOE signal was observed between the H1 (-CH=N-, δ = 7.86 ppm) and H2 (=N-NH-, δ = 11.69 ppm) in the E isomer (E-7a), owing to the large intramolecular H-H distance (Z-7a), NOE signal should not be observed in the putative Z isomer. Thus, target compounds 7a – 7e and 8a – 8g were clearly confirmed as E isomers.
2.3 Synthesis of Benzothiazole-2-carboxylate ( 2 )
Amixture of 2-aminothiophenol (0.05 mol) and ethyl oxalate (0.1 mol) was stirred at 140 °C for 4 h. After completion of reaction as indicated by TLC, the reaction mixture was cooled to room temperature and poured into a solution containing 50 mL of hydrochloric acid, 150 mL of water and 70 mL of ethanol with stirring, the oil dissolved and a solid formed. Then the mixture was separated by filtering under vacuum and washed with water, dried to afford compound 2: white solid; yield 95%; m.p. 68 – 69°C (lit.[35] 68 – 70°C); IR (KBr, cm–1): 1748(C=O), 1500, 1097(C-O), 1012, 722; 1H NMR (DMSO-d6; δ, ppm): 8.29 – 8.19 (m, 2H, ArH), 7.72 – 7.61 (m, 2H, ArH), 4.46 (q, J = 7.0 Hz, 2H, -CH2 -C=O), 1.38 (t, J = 7.1 Hz, 3H, -CH3).
2.4 Synthesis of Benzothiazole-2-carbohydrazide ( 3 )
Compound 2 (0.01 mol) was dissolved in ethanol (60 mL). hydrazine hydrate (80%) (0.02 mol) was added to the solution dropwise with constant stirring and the reaction mixture was refluxed for 9 h. The reaction mixture was cooled to room temperature, filtered, washed with water, dried overnight and finally recrystallized from absolute ethanol, filtered and dried to afford compound 3: light yellow solid; yield 87%; m.p. 173 – 174°C (lit.[36] 172 – 174°C); IR (KBr, cm–1): 3310, 3205 (N-H), 1680 (C=O), 1501, 1201 (C-N), 1022 (N-N), 750; 1H NMR (DMSO-d6; δ, ppm): 10.46 (s, 1H, N-H), 8.22 (d, J = 7.9 Hz, 1H, ArH), 8.11 (d, J = 8.1 Hz, 1H, ArH), 7.62 (t, J = 7.6 Hz, 1H, ArH), 7.57 (t, J = 7.5 Hz, 1H, ArH), 4.72 (s, 2H, NH2).
2.5 Synthesis of Substituted 1H-Indole-3-carbaldehydes ( 5a – 5e )
General procedure. POCl3 (5mL) was dropped into DMF (15mL) in an ice bath and stirred for 30 min. After that, Indole (4a – 4e, 0.01mol) dissolved in 5ml DMF was added into the mixture and heated to 40°C for 1 h. The resulting reaction mixture was poured into 50 mL ice water and then adjusted to pH 8 using 20% NaOH aq. The solution was filtered and the residue was washed with water, dried and finally recrystallized from absolute ethanol, filtered and dried to afford pure compounds 5a – 5e in 95 – 98% yields.
1H-Indole-3-carbaldehyde (5a): orange solid; m.p. 194 – 196°C (lit.[37] 196 – 198°C); 1H NMR (DMSO-d6; δ, ppm): 12.15 (s, 1H, N-H), 9.92 (s, 1H, CHO), 8.27 (s, 1H, ArH), 8.07 (d, J = 7.7 Hz, 1H, ArH), 7.50 (d, J = 8.0 Hz, 1H, ArH), 7.25 (t, J = 7.5 Hz, 1H, ArH), 7.20 (t, J = 7.3 Hz, 1H, ArH).
5-Methoxy-1H-indole-3-carbaldehyde (5b): white solid; m.p. 180 – 182°C (lit.[38] 181 – 182°C).
5-Bromo-1H-indole-3-carbaldehyde (5c): light pink solid; m.p. 206 – 207°C (lit.[37] 202 – 204°C).
5-Chloro-1H-indole-3-carbaldehyde (5d): light yellow solid; m.p. 217 – 218°C (lit.[37] 215 – 216°C).
5-Fluoro-1H-indole-3-carbaldehyde (5e): white solid; m.p. 164 – 165°C (lit.[39] 162 – 163°C).
2.6 Synthesis of Substituted 1-Ethyl-1H-indole-3- carbaldehydes ( 6a – 6c ), Substituted 1-Isopropyl-1Hindole- 3-carbaldehydes ( 6d – 6f ), and 1-(Phenylsulfonyl)- 1H-indole-3-carbaldehyde ( 6g )
General procedure. A mixture of compound 5a, 5b or 5c (0.02 mol) , DMSO (30 mL) and 20% NaOH solution (10 mL) was stirred at r.t. for 15 min. After that, bromoethane, 2-bromopropane or benzenesulfonyl chloride was dropwise added into the mixture and heated to 35°C for 2 – 5 h. After completion of reaction as indicated by TLC, the reaction mixture was cooled to room temperature and poured into water (150 mL), stirring until a solid formed. Filtering under vacuum and washed with water. Recrystallization afforded compound 6a – 6g with yields of 85–94 %.
1-Ethyl-1H-indole-3-carbaldehyde (6a): yellow solid; m.p. 97–99°C (lit.[40] 98–100°C); IR (KBr, cm–1): 3102 (-CH3), 2978 (-CH2-), 1695 (C=O), 1447, 781, 748; 1H NMR (DMSO-d6; δ, ppm): 9.90 (s, 1H, CHO), 8.35 (s, 1H, ArH), 8.11 (d, J = 7.8 Hz, 1H, ArH), 7.64 (d, J = 8.2 Hz, 1H, ArH), 7.32 (t, J = 7.5 Hz, 1H, ArH), 7.26 (t, J = 7.4 Hz, 1H, ArH), 4.31 (q, J = 7.3 Hz, 2H, -CH2-), 1.43 (dd, J = 9.3, 5.2 Hz, 3H, CH3).
1-Ethyl-5-methoxy-1H-indole-3-carboxaldehyde (6b): light pink solid; m.p. 100 – 101°C (lit.[40] 97°C).
5-Bromo-1-ethyl-1H-indole-3-carbaldehyde (6c): white solid; m.p. 106 – 107°C (lit.[40] 98°C).
1-Isopropyl-1H-indole-3-carbaldehyde (6d): yellow solid; m.p. 81 – 83°C; IR (KBr, cm–1): 3120 (-CH3),2984 (CH aliphatic), 1655 (CHO), 1340, 790, 751; 1H NMR (DMSO-d6; δ, ppm): 9.92 (s, 1H, CHO), 8.47 (s, 1H, ArH), 8.11 (d, J = 7.8 Hz, 1H, ArH), 7.68 (d, J = 8.2 Hz, 1H, ArH), 7.33 – 7.29 (m, 1H, ArH), 7.26 (t, J = 7.4 Hz, 1H, ArH), 4.90 – 4.81 (m, 1H, CH aliphatic), 1.52 (d, J = 6.6 Hz, 6H, CH3).
1-Isopropyl-5-methoxy-1H-indole-3-carbaldehyde (6e): yellow solid; m.p. 116 – 117°C.
5-Bromo-1-isopropyl-1H-indole-3-carbaldehyde (6f): light green solid; m.p. 117 – 119°C.
1-(Phenylsulfonyl)-1H-indole-3-carbaldehyde (6g): white solid; m.p. 157 – 158°C; 1H NMR (DMSO-d6; δ, ppm): 10.09 (s, 1H, CHO), 8.91 (s, 1H, phenylsulfonyl), 8.13 (t, J = 6.9 Hz, 3H, phenylsulfonyl), 7.98 (d, J = 8.3 Hz, 1H, phenylsulfonyl), 7.78 (t, J = 7.5 Hz, 1H, ArH), 7.67 (t, J = 7.9 Hz, 2H, ArH), 7.47 (dd, J = 11.5, 4.2 Hz, 1H, ArH), 7.41 (t, J = 7.5 Hz, 1H, ArH).
2.7 Preparation of target compounds ( 7a – 7e and 8a – 8g )
General procedure: A mixture of compound 3 (0.002 mol) and compounds 5a – 5e (6a – 6g) (0.0022 mol) and two drop of glacial acetic acid in ethanol (20 mL) was heated at reflux for 6 h and then cooled to room temperature, the precipitate was filtered and washed with diethyl ether. Finally recrystallized from absolute ethanol and dried in air to furnish pure compounds 7a – 7e and 8a – 8g.
(E)-N′-[(1H-Indol-3-yl)methylene]benzothiazole-2-carbohydrazide (7a): yellow solid; yield 76%; m.p. 278 – 280°C; IR (KBr, cm–1): 3292 (N-H aromatic), 1621 (C=O), 1607, 1544 (C=N), 1235 (C-N), 1127 (N-N), 745; 1H NMR (DMSO-d6; δ, ppm): 12.40 (s, 1H, N-H), 11.69 (s, 1H, =N-NH-), 8.82 (s, 1H, ArH), 8.30 (d, J = 7.9 Hz, 1H, ArH), 8.28 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.86 (d, J = 2.7 Hz, 1H, -CH=N-), 7.67 (t, J = 7.6 Hz, 1H, ArH), 7.62 (t, J = 7.5 Hz, 1H, ArH), 7.46 (d, J = 7.9 Hz, 1H, ArH), 7.23 (t, J = 7.3 Hz, 1H, ArH), 7.18 (t, J = 7.3 Hz, 1H, ArH). 13C NMR (DMSO-d6; δ, ppm): 164.51 (C=O), 155.40, 152.80, 147.43 (C=N-NH-), 137.05, 135.98, 131.05, 127.14, 126.85, 124.33, 123.94, 122.98, 122.73, 121.96, 120.58, 111.87, 111.56.
(E)-N′-[(5-Methoxy-1H-indol-3-yl)methylene]benzothiazole- 2-carbohydrazide (7b): yellow solid; yield 77%; m.p. 270 – 271°C; IR (KBr, cm–1): 3301 (N-H aromatic), 3261 (-CH 3 ), 1671 (C=O), 1608, 1544 (C=N), 1215 (C-N), 1022(N-N), 764; 1H NMR (DMSO-d6; δ, ppm): 12.37 (s, 1H, N-H), 11.55 (s, 1H, =N-NH-), 8.80 (s, 1H, ArH), 8.28 (d, J = 8.0 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.87 (s, 1H, ArH), 7.80 (d, J = 2.6 Hz, 1H, -CH=N-), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.61 (t, J = 7.6 Hz, 1H, ArH), 7.35 (d, J = 8.8 Hz, 1H, ArH), 6.87 (d, J = 8.8 Hz, 1H, ArH), 3.81 (s, 3H, CH3). 13C NMR (DMSO-d6; δ, ppm): 164.58 (C=O), 155.35, 154.56, 152.82, 147.44 (C=N-NH-), 135.99, 132.01, 131.33, 127.12, 126.83, 124.99, 123.92, 122.98, 112.50, 112.36, 111.34, 104.20, 55.28 (O-C).
(E)-N′-[(5-Bromo-1H-indol-3-yl)methylene]benzothiazole- 2-carbohydrazide (7c): yellow solid; yield 82%; m.p. 311 – 313°C; IR (KBr, cm–1): 3204 (N-H aromatic), 1671 (C=O), 1618, 1534 (C=N), 1290 (C-N), 1131 (N-N), 757, 621 (C-Br); 1H NMR (DMSO-d6; δ, ppm): 12.46 (s, 1H, N-H), 11.85 (s, 1H, =N-NH-), 8.77 (s, 1H, ArH), 8.45 (s, 1H, ArH), 8.26 (d, J = 7.9 Hz, 1H, ArH), 8.18 (d, J = 8.1 Hz, 1H, ArH), 7.91 (d, J = 2.4 Hz, 1H, -CH=N-), 7.65 (t, J = 7.3 Hz, 1H, ArH), 7.60 (t, J = 7.6 Hz, 1H, ArH), 7.42 (d, J = 8.6 Hz, 1H, ArH), 7.33 (dd, J = 8.6, 1.8 Hz, 1H, ArH). 13C NMR (DMSO-d6; δ, ppm): 164.35 (C=O), 155.52, 152.80, 146.92 (C=N-NH-), 135.99, 135.80, 132.42, 127.16, 126.90, 125.96, 125.27, 124.15, 123.96, 123.01, 113.97, 113.29, 111.17.
(E)-N′-[(5-Chloro-1H-indol-3-yl)methylene]benzothiazole- 2-carbohydrazide (7d): yellow solid; yield 72%; m.p. 303 – 305°C; IR (KBr, cm–1): 3201 (N-H aromatic), 1671(C=O), 1604, 1544 (C=N), 1291 (C-N), 1131 (N-N), 757, 728(C-Cl); 1H NMR (DMSO-d6; δ, ppm): 12.48 (s, 1H, N-H), 11.87 (s, 1H, =N-NH-), 8.80 (s, 1H, ArH), 8.32 (s, 1H, ArH), 8.28 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.0 Hz, 1H, ArH), 7.95 (d, J = 2.5 Hz, 1H, -CH=N-), 7.67 (t, J = 7.3 Hz, 1H, ArH), 7.62 (t, J = 7.5 Hz, 1H, ArH), 7.48 (d, J = 8.6 Hz, 1H, ArH), 7.24 (d, J = 8.6 Hz, 1H, ArH). 13C NMR (DMSO-d6; δ, ppm): 164.36 (C=O), 155.53, 152.80, 146.91 (C=N-NH-), 135.99, 135.54, 132.57, 127.16, 126.89, 125.33, 125.25, 123.95, 123.00, 122.72, 121.12, 113.50, 111.28.
(E)-N′-[(5-Fluoro-1H-indol-3-yl)methylene]benzothiazole- 2-carbohydrazide (7e): yellow solid; yield 76%; m.p. 294 – 296°C; IR (KBr, cm–1): 3189 (N-H aromatic), 1680 (C=O), 1610, 1544 (C=N), 1317 (C-N), 1288 (C-F), 1044 (N-N), 764; 1H NMR (DMSO-d6; δ, ppm): 12.44 (s, 1H, N-H), 11.77 (s, 1H, =N-NH-), 8.80 (s, 1H, ArH), 8.28 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 8.01 (d, J = 9.7 Hz, 1H, ArH), 7.94 (s, 1H, -CH=N-), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.62 (t, J = 7.5 Hz, 1H, ArH), 7.47 (dd, J = 8.6, 4.5 Hz, 1H, ArH), 7.08 (t, J = 8.2 Hz, 1H, ArH). 13C NMR (DMSO-d6; δ, ppm): 164.40 (C=O), 155.52, 152.80, 146.98 (C=N-NH-), 135.99, 133.66, 132.69, 127.12, 126.85, 123.93, 122.96, 113.05, 111.70, 110.95, 110.75, 106.85, 106.66.
(E)-N′-[(1-Ethyl-1H-indol-3-yl)methylene]benzothiazole-2-carbohydrazide (8a): yellow solid; yield 77%; m.p. 201 – 202°C; IR (KBr, cm–1): 2998 (-CH3), 1648 (C=O), 1608, 1544 (C=N), 1245 (C-N), 1188 (N-N), 762; 1H NMR (DMSO-d6; δ, ppm): 12.38 (s, 1H, =N-NH-), 8.81 (s, 1H, ArH), 8.31 (d, J = 7.8 Hz, 1H, ArH), 8.27 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.94 (s, 1H, -CH=N-), 7.67 (t, J = 7.6 Hz, 1H, ArH), 7.61 (t, J = 7.5 Hz, 1H, ArH), 7.57 (d, J = 8.2 Hz, 1H, ArH), 7.28 (t, J = 7.5 Hz, 1H, ArH), 7.22 (t, J = 7.4 Hz, 1H, ArH), 4.27 (q, J = 7.2 Hz, 2H, -CH2-), 1.42 (t, J = 7.2 Hz, 3H, -CH3). 13C NMR (DMSO-d6; δ, ppm): 164.51 (C=O), 155.38, 152.81, 146.95 (C=N-NH-), 136.60, 135.99, 133.15, 127.14, 126.86, 124.89, 123.93, 122.99, 122.76, 122.21, 120.82, 110.75, 110.28, 40.68, 15.19.
(E)-N′-[(1-Ethyl-5-methoxy-1H-indol-3-yl)methylene]- benzothiazole-2-carbohydrazide (8b): yellow solid; yield 83%; m.p. 172 – 174°C; IR (KBr, cm–1): 2997 (-CH3), 1648 (C=O), 1608, 1544 (C=N), 1255 (C-N), 1185 (N-N), 758; 1H NMR (DMSO-d6; δ, ppm): 12.36 (s, 1H, =N-NH-), 8.78 (s, 1H, ArH), 8.27 (d, J = 8.0 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.87 (s, 2H -CH=N-), 7.67 (t, J = 7.6 Hz, 1H, ArH), 7.61 (t, J = 7.6 Hz, 1H, ArH), 7.48 (d, J = 8.8 Hz, 1H, ArH), 6.92 (d, J = 8.9 Hz, 1H, ArH), 4.22 (q, J = 7.3 Hz, 2H, -CH2-), 3.82 (s, 3H, -CH3), 1.40 (t, J = 7.2 Hz, 3H). 13C NMR (DMSO-d6; δ, ppm): 164.57 (C=O), 155.30, 154.79, 152.81, 146.94 (C=N-NH-), 135.99, 133.30, 131.71, 127.14, 126.84, 125.54, 123.92, 123.00, 112.22, 111.02, 110.34, 104.48, 55.32 (O-C), 40.83, 15.24.
(E)-N′-[(5-Bromo-1-ethyl-1H-indol-3-yl)methylene]- benzothiazole-2-carbohydrazide (8c): yellow solid; yield 76%; m.p. 197 – 199°C; IR (KBr, cm–1): 3102 (-CH3), 1671 (C=O), 1608, 1544 (C=N), 1201 (C-N), 1022 (N-N), 757, 621 (C-Br); 1H NMR (DMSO-d6; δ, ppm): 12.46 (s, 1H, =N-NH-), 8.78 (s, 1H, ArH), 8.48 (s, 1H, ArH), 8.28 (d, J = 8.0 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 8.01 (s, 1H, -CH=N-), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.62 (t, J = 7.3 Hz, 1H, ArH), 7.59 (d, J = 8.7 Hz, 1H, ArH), 7.41 (d, J = 8.5 Hz, 1H, ArH), 4.26 (q, J = 7.1 Hz, 2H, CH2), 1.40 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (DMSO-d6; δ, ppm): 164.35 (C=O), 155.51, 152.79, 146.45 (C=N-NH-), 136.00, 135.41, 134.42, 127.17, 126.90, 126.39, 125.25, 124.36, 123.95, 123.01, 113.61, 112.50, 110.35, 40.93, 15.14.
(E)-N′-[(1-Isopropyl-1H-indol-3-yl)methylene]benzothiazole- 2-carbohydrazide (8d): yellow solid; yield 84%; m.p. 251 – 252°C; IR (KBr, cm–1): 3190 (-CH3), 1657 (C=O), 1648, 1544 (C=N), 1188 (C-N,), 1021 (N-N), 754; 1H NMR (DMSO-d6; δ, ppm): 12.38 (s, 1H, =N-NH-), 8.81 (s, 1H, ArH), 8.31 (d, J = 7.8 Hz, 1H, ArH), 8.27 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.94 (s, 1H, -CH=N-), 7.67 (t, J = 7.6 Hz, 1H, ArH), 7.61 (t, J = 7.5 Hz, 1H, ArH), 7.57 (d, J = 8.2 Hz, 1H, ArH), 7.28 (t, J = 7.5 Hz, 1H, ArH), 7.22 (t, J = 7.4 Hz, 1H, ArH), 4.27 (q, J = 7.2 Hz, 1H, CH), 1.42 (t, J = 7.2 Hz, 6H, CH3). 13C NMR (DMSO-d6; δ, ppm): 164.54 (C=O), 155.41, 152.81, 147.01 (C=N-NH-), 136.45, 135.98, 130.18, 127.14, 126.86, 124.84, 123.94, 122.99, 122.70, 122.24, 120.86, 111.07, 110.40, 47.04, 22.28.
(E)-N′-[(1-Isopropyl-5-methoxy-1H-indol-3-yl)methylene] benzothiazole-2-carbohydrazide (8e): yellow solid; yield 82%; m.p. 220 – 221°C; IR (KBr, cm–1): 3267 (-CH3), 1680 (C=O), 1550, 1544 (C=N), 1203 (C-N), 1023 (N-N), 754; 1H NMR (DMSO-d6; δ, ppm): 12.40 (s, 1H, =N-NH-), 8.79 (s, 1H, ArH), 8.27 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.95 (s, 1H, ArH), 7.89 (d, J = 2.0 Hz, 1H, -CH=N-), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.61 (t, J = 7.5 Hz, 1H, ArH), 7.51 (d, J = 8.9 Hz, 1H, ArH), 6.93 – 6.90 (m, 1H, ArH), 4.76 (dd, J = 13.2, 6.5 Hz, 1H, CH), 3.82 (s, 3H, CH3), 1.49 (d, J = 6.6 Hz, 6H, CH3). 13C NMR (DMSO-d6; δ, ppm): 164.61 (C=O), 155.34, 154.77, 152.82, 147.00 (C=N-NH-), 135.99, 131.54, 130.35, 127.13, 126.84, 125.48, 123.92, 122.99, 112.15, 111.11, 110.64, 104.50, 55.32 (O-C), 47.20, 22.31.
(E)-N′-[(5-Bromo-1-isopropyl-1H-indol-3-yl)methylene] benzothiazole-2-carbohydrazide (8f): yellow solid; yield 84%; m.p. 185 – 187°C; IR (KBr, cm–1): 3067 (-CH3), 1648 (C=O), 1610, 1544 (C=N), 1197 (C-N), 1025 (N-N), 760, 625 (C-Br); 1H NMR (DMSO-d6; δ, ppm): 12.50 (s, 1H, =N-NH-), 8.79 (s, 1H, ArH), 8.49 (s, 1H, ArH), 8.28 (d, J = 7.9 Hz, 1H, ArH), 8.20 (d, J = 8.0 Hz, 1H, ArH), 8.10 (s, 1H, -CH=N-), 7.67 (t, J = 7.5 Hz, 1H, ArH), 7.62 (t, J = 7.1 Hz, 2H, ArH), 7.42 – 7.38 (m, 1H, ArH), 4.81 (dt, J = 13.1, 6.6 Hz, 1H, CH), 1.50 (d, J = 6.6 Hz, 6H, CH3). 13C NMR (DMSO-d6; δ, ppm): 164.37 (C=O), 155.54, 152.80, 146.51 (C=N-NH-), 136.00, 135.23, 131.50, 127.15, 126.89, 126.33, 125.19, 124.40, 123.95, 123.00, 113.59, 112.53, 110.69, 47.42, 22.22.
(E)-N′-[(1-(Phenylsulfonyl)-1H-indol-3-yl)methylene]- benzothiazole-2-carbohydrazide (8g): yellow solid; yield 78%; m.p. 210 – 211°C; IR (KBr, cm–1): 3292, 3055, 1621 (C=O), 1607, 1544 (C=N), 1290 (S=O), 1195 (C-N), 1022 (N-N), 745; 1H NMR (DMSO-d6; δ, ppm): 12.82 (s, 1H, =N-NH-), 8.84 (s, 1H, ArH), 8.42 (d, J = 5.7 Hz, 2H, ArH), 8.28 (d, J = 7.9 Hz, 1H, ArH), 8.22 (d, J = 8.1 Hz, 1H, ArH), 8.09 (d, J = 7.7 Hz, 2H, ArH), 7.99 (d, J = 8.2 Hz, 1H, -CH=N-), 7.74 (t, J = 7.5 Hz, 1H, ArH), 7.68 (t, J = 7.2 Hz, 1H, ArH), 7.63 (td, J = 7.6, 4.3 Hz, 3H, ArH), 7.46 (t, J = 7.4 Hz, 1H, ArH), 7.41 (t, J = 7.5 Hz, 1H, ArH). 13C NMR (DMSO-d6; δ, ppm): 163.80 (C=O), 156.10, 152.73, 144.79 (C=N-NH-), 136.64, 136.04, 134.96, 134.72, 130.53, 129.98, 127.24, 127.07, 126.89, 126.84, 125.95, 124.39, 124.06, 123.29, 123.04, 118.02, 113.08.
3. Experimental Biological Part
By using MTT assay, all target compounds were evaluated for their in vitro antiproliferative activity against Hep G2 (human liver cancer) cells, and sunitinib (a multi-targeted tyrosine kinase inhibitor possessing anti-angiogenic and antitumor activity against a variety of advanced solid tumors) was used as positive controls. The results presented as IC50 values are summarized in Table 2, where the values show average of at least two independent experiments. As can be seen from Table 2, the results indicated that part of the synthesized target compounds showed moderate to excellent cytotoxic activity against Hep G2 cancer cell line. In general, when the C-5 position of indole was substituted by electron- donating group, this was not beneficial to the antiproliferative activity. Notably, compound 7a exhibited the best antiproliferative activity with IC50 values of 0.78 μM for Hep G2 cells, and thus was more potent than the positive control compound sunitinib. Furthermore, electronic and steric effects of the N-1 position of indole were studied by introduction of various R2 groups. The results revealed that increasing size of substituent at the N-1 position of indole clearly decreased the antiproliferative activity (7a vs. 8a, 8d), which suggested that steric hindrance of the group in this region of R2 groups exhibited a negative effect on the cytotoxic activity. On the whole, it seemed that the C-5 substitution of the indole ring of compounds might be crucial for their cytotoxic activities.
In view of above observation, these cytotoxic activity results provided a valuable leading compound 7a with excellent antiproliferative activity, and highlighted the potential for further development of new N-acylhydrazone derivatives as potent antitumor agents.
4. Results and Discussion
In this study, a series of N-acylhydrazone derivatives containing the benzothiazole and indole-based moiety were designed, synthesized and evaluated for the IC50 values against Hep G2 cancer cell line. The relative configuration of target compounds was confirmed as the E isomer. Additionally, we optimized the synthesis of N-substituted indoles to reach a high yield that has not been reported before.
Part of the synthesized target compounds showed moderate to excellent cytotoxic activity. In general, it seemed that the C-5 substitutions of the indole-ring of these compounds might be crucial for their cytotoxic activity. Notably, compound 7a showed better activity against Hep G2 cancer cell line than the positive control drug sunitinib. Thus, compound 7a emerged as a valuable lead for further structural modifications. Further studies on structure optimization are underway in our laboratory. Overall, the structurally new N-acylhydrazone derivatives provide us promising lead compounds for future anticancer drug discovery.
References
F. Bray, J. Ferlay and I. Soerjomataram, CA-Cancer J. Clin., 68(6), 394 – 424 (2018).
J. Shi, P. W. Kantoff, R. Wooster, et al., Nat. Rev. Cancer, 17, 20 – 37 (2017).
H. A. Hamid, A. N. Ramli and M. M. Yusoff, Front. Pharmacol., 8(96), (2017).
P. Ahuja and N. Siddiqui, Eur. J. Med. Chem., 80, 509 – 522 (2014).
S. Mishra, M. Kaur, S. Chander, et al., Eur. J. Med. Chem., 155, 658 – 669 (2018).
M. Zhang, Q. Chen and G. Yang, Eur. J. Med. Chem., 89, 421 – 441 (2015).
Z. Liu, L. Tang, H. Zhu, et al., J. Med. Chem., 59(10), 4637 – 4650 (2016).
K. Chen, Y. Zhang, J. Fan, et al., Eur. J. Med. Chem., 156, 722 – 737 (2018).
E. A. Lafayette, S. Almeida, R. Santos, et al., Eur. J. Med. Chem., 136, 511 – 512 (2017).
U. A. Matulonis, S. Berlin, P. Ivy, et al., J. Clin. Oncol., 27(33), 5601 – 5606 (2009).
M. Nguyen, R. C. Marcellus, A. Roulston, et al., P. Natl. Acad. Sci. Usa., 104(49), 19512 – 19517 (2007).
L. Sun, C. Liang, S. Shirazian, et al., J. Med. Chem., 46(7), 1116 – 1119 (2003).
P. P. Prabhu, S. Pande and C. S. Shastry, Int. J. Chem. Tech. Res., 3(1), 185 – 191 (2011).
S. J. Gilani and S. A. Khan, Med. Chem. Res., 22(7), 3316 – 3328 (2013).
N. H. Cano, M. S. Ballari and A. G. López, et al., J. Agr. Food Chem., 63(14), 3681 – 3686 (2015).
M. Subramanyam, R. Sreenivasulu, R. Gundla, et al., Lett. Drug Des. Discov., 15, 1299 – 1307 (2018).
S. R. Pattan, C. H. Suresh, V. D. Pujar, et al., Indian J. Chem. B., 44, 2404 – 2408 (2005).
S. Murtuja, M. Shaquiquzzaman and M. Amir, Lett. Drug Des. Discov., 15(4), 398 – 405 (2018).
T. Akhtar, S. Hameed, N. Al-Masoudi, et al., Acta. Pharm., 58, 135 – 149 (2008).
F. M. Shaikh, N. B. Patel, G. Sanna, et al., Med. Chem. Res., 24(8), 3129 – 3142 (2015).
S. S. Thakkar, P. Thakor, A. Ray, H. et al., Bioorgan. Med. Chem., 25(20), 5396 – 5406 (2017).
N. D. Amnerkar and K. P. Bhusari, J. Enzyme Inhib. Med. Chem., 26(1), 22 – 28 (2011).
H. Abdel-Aziz, W. Eldehna, M. Fares, et al., Int. J. Mol. Sci., 16(4), 8719 – 8743 (2015).
P. Reddy, Y. Lin and H. Chang, Arkivoc., 16, 113 – 122 (2007).
D. Osmaniye, S. Levent, A. Karaduman, et al., Molecules, 23(5), 1054 – 1067 (2018).
M. Cindriæ, S. Jambon, A. Harej, et al., Eur. J. Med. Chem., 136, 468 – 479 (2017).
C. Zhang, D. Xu, J. Wang, et al., Russ. J. Gen. Chem.+, 87(12), 3006 – 3016 (2017).
J. Ma, G. Bao, L.Wang, et al., Eur. J. Med. Chem., 96, 173 – 186 (2015).
P. Mombelli, M. C. Witschel, A. W. Zijl, et al., Chem. Med. Chem., 7, 151 – 158 (2012).
M. Carcelli, D. Rogolino, A. Gatti, et al., Sci. Rep-UK., 6, 31500 (2016).
A. D. Joshi, R. C. Botham, L. J. Schlein, et al., Oncotarget, 8(46), 80124 – 80138 (2017).
L. P. Figueiredo, A. L. Ibiapino and D. N. Amaral, J. Mol. Struct., 1147, 226 – 234 (2017).
D. A. Rodrigues and G. A. Ferreira-Silva, J. Med. Chem., 59(2), 655 – 670 (2016).
P. C. Lima, L. M. Lima, K. Silva, et al., Eur. J. Med. Chem., 35(2), 187 – 203 (2000).
R. Bhutani, D. P. Pathak, G. Kapoor, et al., Bioorg. Chem., 77, 6 – 1 (2018).
B. Rajeeva, N. Srinivasulu and S. M. Shantakumar, E-J. Chem., 6(3), 775 – 779 (2009).
L. Heda, R. Sharma, C. Pareek and P. B. Chaudhari, E-J. Chem., 6(3), 770 – 774 (2009).
E. Young, J. Chem. Soc., 3493 – 3495 (1958).
J. E. Johnson, D. C. Canseco, D. D. Dolliver, et al., J. Chem. Crystallogr., 39(5), 329 – 336 (2009).
P. K. Dubey and M. Venkatanarayana, Green Chem. Lett. Rev., 3(4), 257 – 261 (2010).
Acknowledgements
The project was funded by the key Laboratory of Marine Bioactive Substances and Modern Analysis Technology, SOA (MBSMAT-2017-06)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, K., Ding, Y. & Kang, C. Synthesis and Antiproliferative Activity of New N-Acylhydrazone Derivatives Containing Benzothiazole and Indole Based Moiety. Pharm Chem J 54, 345–352 (2020). https://doi.org/10.1007/s11094-020-02215-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02215-w