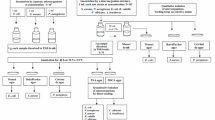
Rapid and correct identification of microorganisms is an essential part of pharmaceutical analysis. Several phenotypic, genotypic, and proteotypic methods, each of which has its own advantages and limitations, are currently used to identify microorganisms. The aim of the present work was to reveal features and validate the operation of a Vitek 2 Compact 30 bacteriological analyzer (bioMerieux, France), which was based on determining the biochemical properties of microorganisms. The applicability of this method was determined using accuracy, precision, and robustness. Correct results were found in 86% of 396 cases. Results obtained under repeatability and intermediate precision conditions showed no differences. Approaches to molecular and genetic identification of microorganisms isolated from preparations in addition to automated biochemical identification were discussed.
Similar content being viewed by others
References
O. V. Gunar, N. G. Sakhno, M. V. Roshchina, V. E. Grigor?eva, et al., Vedom. NTsESMP, 4, 7 – 11 (2014); O. V. Gunar, N. G. Sakhno, M. V. Roshchina, et al., Sci. Centre Expert Eval. Med. Prod. Bull., 4, 7 – 11 (2014).
R. A. Volkova, E. S. Skolotneva, E. V. Elbert, et al., BIOprep. Profil. Diagn. Lech., No. 2, 9 – 14 (2015); R. A. Volkova, E. S. Skolotneva, E. V. Elbert, et al., BIOprep. Prev. Diagn. Treat., No. 2, 9 – 14 (2015); https: // doi.org / 10.30895 /2221-996X-2015-2-9-14.
D. S. Davydov, M. P. Rudnik, A. A. Movsesyants, et al., BIOprep. Profil. Diagn. Lech., No. 4, 52 – 58 (2015); D. S. Davydov, M. P. Rudnik, A. A. Movsesyants, et al., Bioprep. Prev. Diagn. Treat., No. 4, 52 – 58 (2015); https: //doi.org / 10.30895 / 2221-996X-2015-4-52-58.
The United States Pharmacopeia, 40th Ed., The United States Pharmacopeial Convention, Rockville, MD (2017).
J. Hakovirta, Modern Techniques in Detection, Identification and Quantification of Bacteria and Peptides from Foods, Helsinki (2008).
L. Li, N. Mendis, H. Trigui, et al., Front. Microbiol., 5, Article 258 (2014).
T. Sandle, Pharmaceutical Microbiology: Essentials for Quality Assurance and Quality Control, Woodhead Publishing, UK (2015).
G. Funke, D. Monnet, C. de Bernardis, et al., J. Clin. Microbiol., 36(7), 1948 – 1952 (1998).
S. Q. van Veen, E. C. Claas, and E. J. Kuijper, J. Clin. Microbiol., 48, 900 – 907 (2010).
P. C. Schreckenberger, K. L. Ristow, and A. M. Krilcich, Comparison of the Vitek legacy, Vitek 2 colorimetric and Phoenix systems for identification of fermenting and non-fermenting bacteria of clinical origin, 105th General Meeting of the American Society for Microbiology, USA (2005).
U. Eigner, A. Schmid, U. Wild, D. Bertsch, et al., J. Clin. Microbiol., 43(8), 3829 – 3834 (2005).
L. Guo, L. Ye, Q. Zhao, et al., J. Thorac. Dis., 6(5), 534 – 538 (2014).
C. M. O’Hara, F. C. Tenover, and J. M. Miller, J. Clin. Microbiol., 31(12), 3165 – 3169 (1993).
J. A. Odumeru, M. Steele, L. Fruhner, et al., J. Clin. Microbiol., 37(4), 944 – 949 (1999).
S. Sutton, Am. Pharm. Rev., 15(6), 36 – 48 (2012).
FDA. Bacteriological Analytical Manual (BAM), 8th Ed., Rev. A; URL:http: // www.fda.gov / Food / FoodScienceResearch /LaboratoryMethods / ucm2006949.htm.
S. M. Tallent, K. M. Kotewicz, E. A. Strain, and R. W. Bennett, J. AOAC Int., 95(2), 446 – 451 (2012).
N. J. Tourasse, O. A. Okstad, and A. B. Kolsto, Database (2010), Article ID baq017; DOI:10.1093 / database / baq017.
A. Sorokin, B. Candelon, K. Guilloux, et al., Appl. Environ. Microbiol., 72(2), 1569 – 1578 (2006).
M. Sebaihia, M. W. Peck, N. P. Minton, et al., Genome Res., 17(7), 1082 – 1092 (2007); DOI: 10.1101 / gr.6282807.
S. Sutton and A. M. Cundell, Pharmacopeial Forum, 30(5), 1884 – 1894 (2004).
European Pharmacopeia, 9th Ed., Council of Europe, Strasbourg (2017).
PI 0012-3. Recommendations on Sterility Testing, Secretariat of the Pharmaceutical Inspection Convention, Belgium (2007).
B. Malorny, P. T. Tassios, P. Radstrom, et al., Int. J. Food Microbiol., 83, 39 – 48 (2003).
L. Jimenes, S. Smalls, and R. Ignar, J. Microbiol. Methods, 41(3), 259 – 265 (2000).
K. Cankar, D. Stebih, T. Dreo, J. Zel, et al., BMC Biotechnol., 6(37), doi:10.1186 / 1472-6750-6-37 (2006).
Acknowledgments
The work was performed in the framework of State Task for SCEEMP, Ministry of Health of Russia, No. 056-00023-18-02 for applied scientific research (State Contract No. NIR AAAA-A18-118021590049-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 11, pp. 55 – 58, November, 2018.
Rights and permissions
About this article
Cite this article
Gunar, O.V., Sakhno, N.G. Methods of Microbial Identification During Drug Quality Analysis and Applicability Assessment. Pharm Chem J 52, 942–945 (2019). https://doi.org/10.1007/s11094-019-01930-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-01930-3