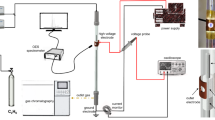
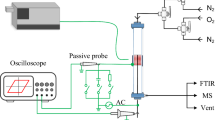
Abstract
Ammonia synthesis via non-thermal plasma presents advantages over the Haber–Bosch process, particularly for small-scale and distributed operations powered by intermittent electricity from renewable energy sources. We designed and characterized a membrane Dielectric-Barrier Discharge (mDBD) reactor for ammonia synthesis from nitrogen and hydrogen. The reactor used a porous alumina membrane as dielectric barrier and as distributor of H2. This arrangement enabled greater residence time for N2 decomposition together with greater H2 availability in the reaction zone, as assessed by a computational thermal-fluid model. We evaluated the reactor's operation with membranes of 0.1, 1.0, and 2.0 µm pore size and porosities between 25 and 51%, and also in conventional DBD mode using a non-porous dielectric. The experimental characterization of the reactor encompassed electrical, optical, and spectroscopic diagnostics, as well as Fourier-Transform Infrared Spectroscopy to analyze gas products, as function of driving voltage. The results show that both, ammonia production and power consumption vary inversely with the product of membrane pore size and porosity. The highest energy yield of 0.25 g-NH3/kWh was obtained with the membrane with 1.0 µm pore size and 35% porosity, whereas the maximum yield under conventional DBD operation was three-times lower. Our findings demonstrate that the use of a membrane dielectric can enhance the performance of DBD-based ammonia synthesis.








Similar content being viewed by others
Data availability
All data that support this research are available upon reasonable request from the authors.
References
Rouwenhorst KHR, Engelmann Y, van‘t Veer K, Postma RS, Bogaerts A, Lefferts L (2020) Plasma-driven catalysis: green ammonia synthesis with intermittent electricity. Green Chem 22(19):6258–6287
Johnson B (2022) Making ammonia: fritz haber, walther nernst, and the nature of scientific discovery. Springer, Berlin
Jennings JR (ed). Catalytic ammonia synthesis: fundamentals and practice. Springer;1991.
Shah JR, Gorky F, Lucero J, Carreon MA, Carreon ML (2020) Ammonia synthesis via atmospheric plasma catalysis: zeolite 5A, a case of study. Ind Eng Chem Res 59(11):5167–5176
Carreon ML (2019) Plasma catalytic ammonia synthesis: state of the art and future directions. J Phys D Appl Phys 52(48):4801
Singh AR, Rohr BA, Schwalbe JA, Cargnello M, Chan K, Jaramillo TF, Chorkendorff Ib, Nørskov JK (2017) Electrochemical ammonia synthesis: the selectivity challenge. ACS Catal 7(1):706–709
Banerjee A, Yuhas BD, Margulies EA, Zhang Y, Shim Y, Wasielewski MR, Kanatzidis MG (2015) Photochemical nitrogen conversion to ammonia in ambient conditions with FeMoS-chalcogels. J Am Chem Soc 137(5):2030–2034
Rouwenhorst KHR, Jardali F, Bogaerts A, Lefferts L (2021) From the Birkeland-Eyde process towards energy-efficient plasma-based NOx synthesis: a techno-economic analysis. Energy Environ Sci 14(5):2520–2534
Brewer AK, Westhaver JW (2002) The synthesis of ammonia in the glow discharge. J Phys Chem 33(6):883–895
Kim M, Biswas S, Nava G, Wong BM, Mangolini L (2022) Reduced energy cost of ammonia synthesis via RF plasma pulsing. ACS Sustain Chem Eng 10(46):15135–15147
Shah J, Wang W, Bogaerts A, Carreon ML (2018) Ammonia synthesis by radio frequency plasma catalysis: revealing the underlying mechanisms. ACS Appl Energy Mater 1(9):4824–4839
Araia A, Wang Y, Robinson B, Jiang C, Brown S, Wildfire C, Shekhawat D, Jianli Hu (2022) Microwave-assisted ammonia synthesis over Cs-Ru/CeO2 catalyst at ambient pressure: Effects of metal loading and support particle size. Catal Commun 170:106491
Hong J, Prawer S, Murphy AB (2014) Production of ammonia by heterogeneous catalysis in a packed-bed dielectric-barrier discharge: influence of argon addition and voltage. IEEE Trans Plasma Sci 42(10):2338–2339
Kumari S, Pishgar S, Schwarting ME, Paxton WF, Spurgeon JM (2018) Synergistic plasma-assisted electrochemical reduction of nitrogen to ammonia. Chem Commun 54(95):13347–13350
Zhou D, Zhou R, Zhou R, Liu B, Zhang T, Xian Y, Cullen PJ, Lu X, Ostrikov KK (2021) Sustainable ammonia production by non-thermal plasmas: Status, mechanisms, and opportunities. Chem Eng J 421:129544
Bogaerts A, Tu X, Whitehead JC, Centi G, Lefferts L, Guaitella O, Azzolina-Jury F et al (2020) The 2020 plasma catalysis roadmap. J Phys D Appl Phys 53(44):443001
Mizushima T, Matsumoto K, Sugoh J-I, Ohkita H, Kakuta N (2004) Tubular membrane-like catalyst for reactor with dielectric-barrier-discharge plasma and its performance in ammonia synthesis. Appl Catal A 265(1):53–59
Carreon ML (2019) Plasma catalysis: a brief tutorial. Plasma Res Express 1(4):043001
Hong J, Prawer S, Murphy AB (2018) Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain Chem Eng 6(1):15–31
Peng P, Li Y, Cheng Y, Deng S, Chen P, Ruan R (2016) Atmospheric pressure ammonia synthesis using non-thermal plasma assisted catalysis. Plasma Chem Plasma Process 36:1201–1210
Xie Q, Zhuge S, Song X, Meizhen Lu, Fengwen Yu, Ruan R, Nie Y (2019) Non-thermal atmospheric plasma synthesis of ammonia in a DBD reactor packed with various catalysts. J Phys D Appl Phys 53(6):064002
Hattori M, Iijima S, Nakao T, Hosono H, Hara M (2020) Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 ℃. Nat Commun 11(1):2001
Okubo M (2022) Recent development of technology in scale-up of plasma reactors for environmental and energy applications. Plasma Chem Plasma Process 42(1):3–33
Pârvulescu VI, Magureanu M, Lukes P (2012) Plasma chemistry and catalysis in gases and liquids. Wiley, New York
Kogelschatz U, Eliasson B, Egli W (1997) Dielectric-barrier discharges: Principle and applications. Le Journal de Physique IV 7(C4):C4-47
Fridman A (2008) Plasma chemistry. Cambridge University Press, Cambridge
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46
Kogelschatz U, Eliasson B, Egli W (1999) From ozone generators to flat television screens: history and future potential of dielectric-barrier discharges. Pure Appl Chem 71(10):1819–1828
Kogelschatz U (2002) Filamentary, patterned, and diffuse barrier discharges. IEEE Trans Plasma Sci 30(4):1400–1408
Eliasson B, Kogelschatz U (1991) Modeling and applications of silent discharge plasmas. IEEE Trans Plasma Sci 19(2):309–323
Eliasson B, Kogelschatz U (1991) Nonequilibrium volume plasma chemical processing. IEEE Trans Plasma Sci 19(6):1063–1077
Rouwenhorst KHR, Travis AS, Lefferts L (2022) 1921–2021: A Century of renewable ammonia synthesis. Sustain Chem 3(2):149–171
Gorbanev Y, Engelmann Y, van’t Veer, Vlasov E, Ndayirinde C, Yi Y, Bals S, Bogaerts A (2021) Al2O3-supported transition metals for plasma-catalytic NH3 synthesis in a DBD plasma: metal activity and insights into mechanisms. Catalysts 11(10):1230
Iwamoto M, Akiyama M, Aihara K, Deguchi T (2017) Ammonia synthesis on wool-like Au, Pt, Pd, Ag, or Cu electrode catalysts in nonthermal atmospheric-pressure plasma of N2 and H2. ACS Catal 7(10):6924–6929
van ‘t Veer K, Engelmann Y, Reniers F, Bogaerts A (2020) Plasma-catalytic ammonia synthesis in a DBD plasma: role of microdischarges and their afterglows. J Phys Chem C 124(42):22871–22883
Gershman S, Fetsch H, Gorky F, Carreon ML (2022) Identifying regimes during plasma catalytic ammonia synthesis. Plasma Chem Plasma Process 42(4):731–757
Barboun P, Mehta P, Herrera FA, Go DB, Schneider WF, Hicks JC (2019) Distinguishing plasma contributions to catalyst performance in plasma-assisted ammonia synthesis. ACS Sustain Chem Eng 7(9):8621–8630
Mizushima T, Matsumoto K, Ohkita H, Kakuta N (2007) Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge. Plasma Chem Plasma Process 27:1–11
Carreon M, Gorky F, Nguyen HM, Lucero JM, Guthrie S, Crawford JM, Carreon MA. Porous Organic Cage Membranes for the Non-Thermal Plasma Catalytic Blue Ammonia Synthesis: The Protective Role of Porous Organic Cage Cc3. https://ssrn.com/abstract=4060684 or https://doi.org/10.2139/ssrn.4060684.
Gómez-Ramírez A, Cotrino J, Lambert RM, González-Elipe AR (2015) Efficient synthesis of ammonia from N2 and H2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci Technol 24(6):065011
Nagy E (2018) Basic equations of mass transport through a membrane layer. Elsevier, New York
Keil FJ (2018) Process intensification. Rev Chem Eng 34(2):135–200
Wang X, Guo W, Shanshan Xu, Chen H, Fan X (2023) Stainless steel membrane distributor-type dielectric barrier discharge plasma reactor for co-conversion of CH4/CO2. AIChE J 69(7):e18059
Mays TJ. A new classification of pore sizes. Studies in surface science and catalysis 160, no. Characterization of Porous Solids VII (2007);57–62.
Espinal L (2002) Porosity and its measurement. Charact Mater 1:1–10
Liu J, Zhu X, Xueli Hu, Zhang F, Xin Tu (2022) Plasma-assisted ammonia synthesis in a packed-bed dielectric barrier discharge reactor: effect of argon addition. Vacuum 197:110786
i.materialize. Accessed: June 12, 2023 (Online). https://i.materialise.com
Solidworks Flow Simulation (2021) Dassault Systèmes SolidWorks Corporation. November 15, 2022 (Online). https://www.solidworks.com/domain/simulation
Gorky F, Nguyen HM, Lucero JM, Guthrie S, Crawford JM, Carreon MA, Carreon ML (2022) CC3 porous organic cage crystals and membranes for the non-thermal plasma catalytic ammonia synthesis. Chem Eng J Adv 11:100340
Nishimura Advanced Ceramics Co., Ltd (2023) Accessed: June 27, 2023 (Online). https://nishimuraac.com/.
Bogaerts A, Zhang Q-Z, Zhang Y-R, Van Laer K, Wang W (2019) Burning questions of plasma catalysis: answers by modeling. Catal Today 337:3–14
Ohno N, Abdur Razzak M, Ukai H, Takamura S, Uesugi Y (2006) Validity of electron temperature measurement by using Boltzmann plot method in radio frequency inductive discharge in the atmospheric pressure range. Plasma Fus Res 1:028–028
Gordillo-Vázquez FJ, Camero M, Gómez-Aleixandre C (2005) Spectroscopic measurements of the electron temperature in low pressure radiofrequency Ar/H2/C2H2 and Ar/H2/CH4 plasmas used for the synthesis of nanocarbon structures. Plasma Sources Sci Technol 15(1):42
Kais A, Lo J, Thérèse L, Guillot P (2018) Heating power at the substrate, electron temperature, and electron density in 2.45 GHz low-pressure microwave plasma. Phys Plasmas 25(1):1
Vecten S, Wilkinson M, Bimbo N, Dawson R, Herbert BMJ (2021) Hydrogen-rich syngas production from biomass in a steam microwave-induced plasma gasification reactor. Bioresource Technol 337:125324
Dalton GR, Dragoset RA, Fuhr JR, Kelleher DE, Kotochigova SA, Martin WC, Mohr PJ, Musgrove A, Olsen Podobedova KL (1998) NIST atomic spectra database. NIST Spec Publ 1:12–15
Hong Yi, Niu J, Pan J, Bi Z, Ni W, Liu D, Li J, Yan Wu (2016) Electron temperature and density measurement of a dielectric barrier discharge argon plasma generated with tube-to-plate electrodes in water. Vacuum 130:130–136
Subedi DP, Tyata RB, Shrestha R, Wong CS. An experimental study of atmospheric pressure dielectric barrier discharge (DBD) in argon. In AIP Conference Proceedings, vol. 1588, no. 1, pp. 103–108. American Institute of Physics;2014.
Tabu B, Veng V, Morgan H, Das SK, Brack E, Alexander T, Hunter Mack J, Wong H-W, Trelles JP. Hydrogen from cellulose and low-density polyethylene via atmospheric pressure nonthermal plasma. Int J Hydrog Energy (2023).
Hong J, Aramesh M, Shimoni O, Seo DH, Yick S, Greig A, Charles C, Prawer S, Murphy AB (2016) Plasma catalytic synthesis of ammonia using functionalized-carbon coatings in an atmospheric-pressure non-equilibrium discharge. Plasma Chem Plasma Process 36:917–940
Funding
Department of Navy award N00014-22-1-2001 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world for all copyrightable material contained herein.
Author information
Authors and Affiliations
Contributions
VV Investigation, Visualization, Writing—Original Draft Preparation; BT Investigation; ES Methodology; JEL Investigation; JHM Resources and Supervision; MC Conceptualization and Supervision; JPT Conceptualization, Funding Acquisition, Project Administration, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Veng, V., Tabu, B., Simasiku, E. et al. Design and Characterization of a Membrane Dielectric-Barrier Discharge Reactor for Ammonia Synthesis. Plasma Chem Plasma Process 43, 1921–1940 (2023). https://doi.org/10.1007/s11090-023-10402-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10402-2