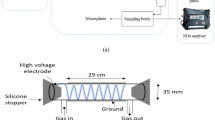
Abstract
Effects of C3H6 and CO2 on desulfurization and denitrification by nonthermal plasma (NTP) and formation of the by-product CO were investigated under the simulated diesel exhaust condition. Optical emission spectroscopy (OES) was employed to observe the plasma process, and effects of C3H6 and CO2 on the emission intensities of O \({\text{(3p}}^{{5}} {\text{P}} \to {\text{3s}}^{{5}} {\text{S}}_{{2}}^{{0}} {)}\) and OH \(({A}^{2}{\sum }^{+}\to {\mathrm{X}}^{2}\prod )\) were detected. The experimental result shows that the CO2 concentration has negligible effect on formation of O and OH radicals, and the change in the CO2 concentration has no significant impact on the removal of NO and SO2, but every 1% increase of the CO2 concentration will raise the CO by about 22.5 ppm. When the C3H6 concentration increases from 0 to 600 ppm, the NO removal efficiency increases from 42.8 to 70.5%. However, the existence of C3H6 has marginal effect on the SO2 removal. C3H6 is an effective additive for the oxidative removal of NO, which can react with O and OH radicals in DBD reactor and generate a lot of oxidative radicals including HO2 and RO2 (C2H5O2, CH3O2, HOC3H6O2). The generated HO2 and RO2 will replace O and OH radicals as the main species to realize the oxidative removal of NO. However, the by-product CO formed in the plasma process and the unreacted C3H6 also need to be concerned. Through analyzing the mechanism of CO2 and C3H6 promoting the removal of NO and SO2 in a more realistic simulated gas atmosphere based on OES, the present study can provide guidance for improving the efficiencies of desulfurization and denitrification for marine diesel exhaust by NTP.












Similar content being viewed by others
References
Cengiz D, Burak Z (2016) J Clean Prod 113:438. https://doi.org/10.1016/j.jclepro.2015.11.089
Wang ZY et al (2019) RSC Adv 9:5402. https://doi.org/10.1039/c8ra09217f
Boone L (2012) Carb Clim Law Rev 6(1):13. https://doi.org/10.21552/CCLR/2012/1/204
Francesco DN, Claudia C (2015) Transp Res Part D Transp Environ 40(14):166. https://doi.org/10.1016/j.trd.2015.08.011
Fang P et al (2017) Chem Indus Eng Prog 36(3):1067
IMO, 2019a Nitrogen oxides (NOx)-regulation 13. Accessed 21 Feburary 2019. https://www.imo.org/en/ourwork/environment/pollutionprevention/airpollution/pages/nitrogen-oxides-(nox)-%E2%80%93-regulation-13.aspx
IMO, 2019b Sulphur Oxides (SOx) and particulate matter (PM)-regulation 14. Accessed 22 Feburary 2019. https://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/Sulphur-oxides-(SOx)-%E2%80%93-Regulation-14.aspx
Feng T, Lü L (2015) J Ind Eng Chem 28:97. https://doi.org/10.1016/j.jiec.2015.02.004
Chen S et al (2019) Fuel Process Technol 186:125. https://doi.org/10.1016/j.fuproc.2018.12.022
Dean B, Radoslav R (2011) Sci J Marit Res 25(1):15
Fang P et al (2011) Chem Eng J 168(1):52. https://doi.org/10.1016/j.cej.2010.12.030
Jolibois J, Takashima K, Mizuno A (2012) J Electrostat 70(3):300. https://doi.org/10.1016/j.elstat.2012.03.011
Kuroki T et al (2001) IEEE Trans Ind Appl 38(5):1204. https://doi.org/10.1109/TIA.2002.802919
Wang MY, Sun YF, Zhu TL (2013) IEEE Trans Plasma Sci 41(2):312. https://doi.org/10.1109/TPS.2012.2234483
Ma S et al (2017) Renew Sustain Energy Rev 67:791. https://doi.org/10.1016/j.rser.2016.09.066
Mizuno A et al (1995) IEEE Trans Ind Appl 31(5):957. https://doi.org/10.1109/28.464504
Kim HH et al (2001) J Phys D Appl Phys 34(4):604. https://doi.org/10.1088/0022-3727/34/4/322
Mok YS et al (2000) Ind Eng Chem Res 39(10):3938. https://doi.org/10.1021/ie000239o
Shang K, Wu Y (2009) 2009 3rd international conference on bioinformatics and biomedical engineering, Beijing, China. https://doi.org/10.1109/icbbe.2009.5162261
Cai YX et al (2010) Plasma Sci Technol 12:482. https://doi.org/10.1088/1009-0630/12/4/19
Schmidt M, Basner R, Brandenburg R (2013) Plasma Chem Plasma Process 33(1):323. https://doi.org/10.1007/s11090-012-9424-6
Li XH et al (2013) Environ Chem 12:2297. https://doi.org/10.7524/j.issn.0254-6108.2013.12.011
Wang T et al (2012) SIESC J 11:285. https://doi.org/10.3969/j.issn.0438-1157.2012.11.040
Zhang X et al (2016) React Eng Technol 6:553
Liu F (2018) Experimental study on the oxidation of NO by non-thermal Plasma. Master thesis, Wuhan University of Technology (in Chinese)
Cai YK, Lv L, Lu XP (2021) High Volt 6(6):1092
Cai YK, Lv L, Li P (2020) Appl Sci 10(19):6766. https://doi.org/10.3390/app10196766
Eichwald O et al (1997) J Appl Phys 82(10):4781. https://doi.org/10.1063/1.366336
Shin HH, Yoon WS (2003) Plasma Chem Plasma Process 23(4):681. https://doi.org/10.1023/A:1025595318945
Zhu TL (2018) Removal of nitrogen oxides and fog particles from wet flue gas desulfurization exhaust by non-thermal plasma. NSFC shared service network (in Chinese). http://output.nsfc.gov.cn/conclusionProject/21377009
Paris P et al (2005) J Phys D Appl Phys 38(21):3894. https://doi.org/10.1088/0022-3727/38/21/010
Cai YK, Lv L, Lu XP (2021) IEEE Trans Plasma Sci 49(2):786. https://doi.org/10.1109/TPS.2021.3049126
Chae JO (2003) J Electrostat 57:251. https://doi.org/10.1016/S0304-3886(02)00165-1
Wang XC, Shang KF (2009) High Volt Eng 35(5):1122
Acknowledgements
This research was funded by the Natural Science Foundation of Hubei Province of China (grant number 2022CFB730). And, I would like to thank Professor Lu Xinpei of Huazhong University of Science and Technology for his guidance and help in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Xiang, C., Zhu, N. et al. Experimental Study on Influences of C3H6 and CO2 in Diesel Exhaust on Desulfurization and Denitrification by Nonthermal Plasma. Plasma Chem Plasma Process 43, 619–633 (2023). https://doi.org/10.1007/s11090-023-10321-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10321-2