Abstract
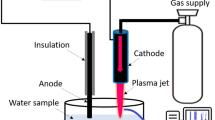
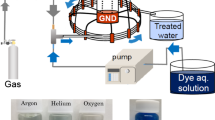
Oxidizing species generated during the plasma discharges in gas–water mixture are well reported for their potential applications in water disinfection. Ozone is an important oxidizing agent produced in these non-thermal plasmas. Present paper reports the spectrochemical analysis of ozone generated during pulsed plasma discharge in oxygen–water mixture. Ozone density in the plasma discharge channel was estimated by using absorption of incident ultraviolet radiation of a light emitting diode at 253.6 nm in the Hartley band of ozone. Based on UV absorption, the peak ozone density noted in the discharge channel was \((1.764 \pm 0.11) \times {10}^{16}/{\text{cm}}^{3}\). While the post plasma ozone density in the effluent water samples was determined through indigo method. The peak ozone density obtained by using this chemical probe was \((1.209 \pm 0.052) \times {10}^{16}/{\text{cm}}^{3}\), showing post plasma ozone depletion along the water flow. Moreover, ozone concentration was highly influenced by average discharge power and gas temperature and showed a decreasing trend with increase in these parameters. Presently, plasma discharge was also generated in aqueous solution of indigo trisulfonate and the resultant decolorization of known concentration of this dye was used to estimate the density of different oxidants in the discharge channel. Results showed that the estimated peak oxidant density in the discharge channel was \((3.818 \pm 0.087) \times 1{0}^{16}/{\text{cm}}^{3}\). While the energy yield of the plasma discharge for decolorization of indigo trisulfonate was \((148.905 \pm 16.314)\text{ g}/\text{kWh}\) with indigo decolorization efficiency of 79.261 ± 1.842%.











Similar content being viewed by others
References
Zhang Y, Wei L, Liang X, Deng H, Šimek M (2018) Characteristics of the discharge and ozone generation in oxygen-fed coaxial DBD using an amplitude-modulated AC power supply. Plasma Chem Plasma Process 38:1199–1208
Kogelschatz U (2017) Ozone and beyond: the marvelous development of dielectric barrier discharges. J Phys D Appl Phys 50:051001
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46
Pekárek S, Babchenko O, Mikeš J, Kromka A (2020) Effect of a diamond layer on the active electrode on the ozone generation of the dielectric barrier discharge in air. J Phys D Appl Phys 53:275203
Pandiselvam R, Sunoj S, Manikantan M, Kothakota A, Hebbar K (2017) Application and kinetics of ozone in food preservation. Ozone Sci Eng 39:115–126.
Malik MA, Hughes D, Heller R, Schoenbach KH (2015) Surface plasmas versus volume plasma: energy deposition and ozone generation in air and oxygen. Plasma Chem Plasma Process 35:697–704
Šimek M, Pekárek S, Prukner V (2010) Influence of power modulation on ozone production using an AC surface dielectric barrier discharge in oxygen. Plasma Chem Plasma Process 30:607–617
Jodzis S (2012) Effective ozone generation in oxygen using a mesh electrode in an ozonizer with variable linear velocity. Ozone Sci Eng 34:378–386.
Kim M, Cho J, Ban S, Choi R, Kwon E, Park S, Eden J (2013) Efficient generation of ozone in arrays of microchannel plasmas. J Phys D Appl Phys 46:305201
Malik MA, Schoenbach KH (2014) Nitric oxide conversion and ozone synthesis in a shielded sliding discharge reactor with positive and negative streamers. Plasma Chem Plasma Process 34:93–109
Malik MA (2014) Ozone synthesis using shielded sliding discharge: effect of oxygen content and positive versus negative streamer mode. Ind Eng Chem Res 53:12305–12311
Malik MA, Jiang C, Dhali SK, Heller R, Schoenbach KH (2014) Coupled sliding discharges: a scalable nonthermal plasma system utilizing positive and negative streamers on opposite sides of a dielectric layer. Plasma Chem Plasma Process 34:871–886
Huang W, Ren T, Xia W (2007) Ozone generation by hybrid discharge combined with catalysis. Ozone Sci Eng 29:107–112.
Liang Chen H, Ming Lee H, Been Chang M (2006) Enhancement of energy yield for ozone production via packed-bed reactors. Ozone Sci Eng 28:111–118.
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42:053001
Tijani JO, Fatoba OO, Madzivire G, Petrik LF (2014) A review of combined advanced oxidation technologies for the removal of organic pollutants from water. Water Air Soil Pollut 225:1–30
Bingyan C, Changping Z, Juntao F, Xiang H, Cheng Y, Yuan W, Ying G, Yongfeng J, Wen W, Longwei C (2016) Yield of ozone, nitrite nitrogen and hydrogen peroxide versus discharge parameter using APPJ under water. Plasma Sci Technol 18:278
Khan MSI, Lee E-J, Kim Y-J (2015) Roles of individual radicals generated by a submerged dielectric barrier discharge plasma reactor during Escherichia coli O157: H7 inactivation. AIP Adv 5:107111
Sun Y, Qiu Y, Nie A, Wang X (2007) Experimental research on inactivation of bacteria by using dielectric barrier discharge. IEEE Trans Plasma Sci 35:1496–1500
Jiang B, Zheng J, Qiu S, Wu M, Zhang Q, Yan Z, Xue Q (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368
Chen Z, Krasik YE, Cousens S, Ambujakshan AT, Corr C, Dai XJ (2017) Generation of underwater discharges inside gas bubbles using a 30-needles-to-plate electrode. J Appl Phys 122:153303
Sharma A, Camaioni D, Josephson G, Goheen S, Mong G (1997) Formation and measurement of ozone and nitric acid in a high voltage DC negative metallic point-to-aqueous-plane continuous corona reactor. J Adv Oxidation Technol 2:239–247
Ihara S, Miichi T, Satoh S, Yamabe C, Sakai E (1999) Ozone generation by a discharge in bubbled water. Jpn J Appl Phys 38:4601
Malik MA, Ghaffar A, Malik SA (2001) Water purification by electrical discharges. Plasma Sources Sci Technol 10:82
Lee E-J, Kim J-S, Jeong M-C, Kim Y-J (2016) Application of underwater dielectric barrier discharge as a washing method for reduction of Salmonella Typhimurium on perilla leaves. LWT-Food Sci Technol 69:146–152
Wei C, Zhang F, Hu Y, Feng C, Wu H (2017) Ozonation in water treatment: the generation, basic properties of ozone and its practical application. Rev Chem Eng 33:49–89
Locke BR, Shih K-Y (2011) Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci Technol 20:034006
Gupta SB (2007) Investigation of a physical disinfection process based on pulsed underwater corona discharges, in, Forschungszentrum Karlsruhe.
Bader H, Hoigné J (1981) Determination of ozone in water by the indigo method. Water Res 15:449–456
Hodges J, Viallon J, Brewer P, Drouin B, Gorshelev V, Janssen C, Lee S, Possolo A, Smith M, Walden J (2019) Recommendation of a consensus value of the ozone absorption cross-section at 253.65 nm based on a literature review. Metrologia 56:034001.
Degner M, Damaschke N, Ewald H, O'keeffe S, Lewis E (2009) UV LED-based fiber coupled optical sensor for detection of ozone in the ppm and ppb range. In: SENSORS, 2009 IEEE. IEEE, New York, pp 95–99.
Wijaikhum A, Schröder D, Schröter S, Gibson AR, Niemi K, Friderich J, Greb A, Schulz-von der Gathen V, O’Connell D, Gans T (2017) Absolute ozone densities in a radio-frequency driven atmospheric pressure plasma using two-beam UV-LED absorption spectroscopy and numerical simulations. Plasma Sources Sci Technol 26:115004
Wang J, Liu H, Wang Y, Ma D, Yao G, Yue Q, Gao B, Xu X (2022) A new UV source activates ozone for water treatment: Wavelength-dependent ultraviolet light-emitting diode (UV-LED). Sep Purif Technol 280:119934
Kim YH, Hong YJ, Baik KY, Kwon GC, Choi JJ, Cho GS, Uhm HS, Choi EH (2014) Measurement of reactive hydroxyl radical species inside the biosolutions during non-thermal atmospheric pressure plasma jet bombardment onto the solution. Plasma Chem Plasma Process 34:457–472
Dorn H, Neuroth R, Hofzumahaus A (1995) Investigation of OH absorption cross sections of rotational transitions in the A2S+, v’= 0<–X2P, v’’= 0 band under atmospheric conditions: Implications for tropospheric long-path absorption. J Geophys Res D-Atmos-Printed Edition 100:7397–7410
Gorshelev V, Serdyuchenko A, Weber M, Chehade W, Burrows J (2014) High spectral resolution ozone absorption cross-sections–Part 1: Measurements, data analysis and comparison with previous measurements around 293 K. Atmos Meas Techn 7:609–624
Andersen PC, Williford CJ, Birks JW (2010) Miniature personal ozone monitor based on UV absorbance. Anal Chem 82:7924–7928
Burrows JP, Richter A, Dehn A, Deters B, Himmelmann S, Voigt S, Orphal J (1999) Atmospheric remote-sensing reference data from GOME—2. Temperature-dependent absorption cross sections of O3 in the 231–794 nm range. J Quant Spectrosc Radiat Transf 61:509–517.
Lai J, Foster JE (2019) Plasma-driven reactive species production and transport in a 2-D discharge cell. IEEE Trans Plasma Sci 47:4422–4427
García MC, Mora M, Esquivel D, Foster JE, Rodero A, Jiménez-Sanchidrián C, Romero-Salguero FJ (2017) Microwave atmospheric pressure plasma jets for wastewater treatment: degradation of methylene blue as a model dye. Chemosphere 180:239–246
Rashid M, Chowdhury M, Talukder M (2020) Textile wastewater treatment by underwater parallel-multi-tube air discharge plasma jet. J Environ Chem Eng 8:104504
Wu L, Xie Q, Lv Y, Wu Z, Liang X, Lu M, Nie Y (2019) Degradation of methylene blue via dielectric barrier discharge plasma treatment. Water 11:1818
Lukes P, Clupek M, Babicky V, Sunka P (2008) Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci Technol 17:024012
Xiong R, Nikiforov A, Vanraes P, Leys C (2012) Hydrogen peroxide generation by dc and pulsed underwater discharge in air bubbles. J Adv Oxid Technol 15:197–204
Akiyama H (2000) Streamer discharges in liquids and their applications. IEEE Trans Dielectr Electr Insul 7:646–653
Bruggeman P, Iza F, Guns P, Lauwers D, Kong MG, Gonzalvo YA, Leys C, Schram DC (2009) Electronic quenching of OH (A) by water in atmospheric pressure plasmas and its influence on the gas temperature determination by OH (A–X) emission. Plasma Sources Sci Technol 19:015016
Goldman A, Gillis JR (1981) Spectral line parameters for the A2∑-X2Π (0, 0) band of OH for atmospheric and high temperatures. J Quant Spectrosc Radiat Transfer 25:111–135
Dieke G, Crosswhite H (2010) The ultraviolet bands of OH fundamental data. J Quant Spectrosc Radiat Transfer 111:1516–1542
Sarani A, Nikiforov AY, Leys C (2010) Atmospheric pressure plasma jet in Ar and Ar/H 2 O mixtures: Optical emission spectroscopy and temperature measurements. Phys Plasmas 17:063504
Hong Y, Nam C, Song K, Cho G, Uhm H, Choi D, Choi E (2012) Measurement of hydroxyl radical density generated from the atmospheric pressure bioplasma jet. J Instrum 7:C03046
Yehia A (2007) Calculation of ozone generation by positive dc corona discharge in coaxial wire-cylinder reactors. J Appl Phys 101:023306
Staehelin J, Hoigne J (1982) Decomposition of ozone in water: rate of initiation by hydroxide ions and hydrogen peroxide. Environ Sci Technol 16:676–681
Anbar M, Meyerstein D, Neta P (1966) The reactivity of aromatic compounds toward hydroxyl radicals. J Phys Chem 70:2660–2662
Acknowledgements
The authors would like to thank Higher Education Commission (HEC), Islamabad, Pakistan for the financial support of the work under the Research Project No. TDF-137.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farooq, M., Khan, M.I. & Rehman, N.U. Spectrochemical Analysis of Ozone Density for Pulsed Plasma Discharge in Oxygen–Water Mixture. Plasma Chem Plasma Process 42, 785–800 (2022). https://doi.org/10.1007/s11090-022-10260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-022-10260-4