Abstract
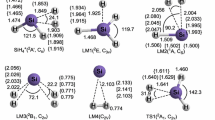
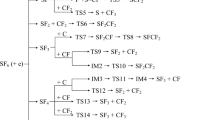
The mechanisms and kinetics for the thermal decomposition of SiH2+ and SiH3+ ions, and related reverse reactions involving their ion fragments have been investigated for the first time by using ab initio molecular orbital and variational RRKM calculations. Geometries of all species involved the title reactions have been optimized by the CCSD(T), CCSD, and MP2 methods with the Aug-CC-pVTZ basis set while their single point energies have been refined by the CCSD(T)/CBS calculation for all the three levels for comparison. The barrierless processes involved have been calculated at the CASPT2//CASSCF/Aug-CC-pVTZ level of theory. The potential energy surfaces indicate that the SiH2+ and SiH3+ ions can eliminate an H atom via barrierless processes with dissociation energies of 48.4 and 91.5 kcal mol−1, respectively, or eliminate an H2 molecule via tight transition states at TS1 (38.5 kcal mol−1) and TS3 (59.9 kcal mol−1) giving H2 + Si+ (21.3 kcal mol−1) and H2 + SiH+ (36.7 kcal mol−1), respectively, at the CCSD(T)/CBS//CCSD(T)/Aug-CC-pVTZ level of theory. The rate constants for the reactions predicted for a wide range of T,P-conditions reveal that they increase with rising temperature and pressure and H2 elimination is the major channel in each of the decomposition reactions. The relative energies predicted by different methods agree closely; the predicted heats of formation for various species are in good agreement with available experimental values. The predicted rate constants for the forward and reverse reactions may be employed for kinetic modeling of PECVD processes.



Similar content being viewed by others
References
Nishizaki S, Ohdaira K, Matsumura H (2009) Comparison of a-Si TFTs fabricated by Cat-CVD and PECVD methods. Thin Solid Films 517:3581–3583
Ay F, Aydinli A (2004) Comparative investigation of hydrogen bonding in silicon based PECVD grown dielectrics for optical waveguides. Opt Mater 26:33–46
Iliescu C, Chen BT (2008) Thick and low-stress PECVD amorphous silicon for MEMS applications. J Micromech Microeng 18:015024
Liu L, Liu WG, Cao N, Cai CL (2013) Study on the performance of PECVD silicon nitride thin films. Def Technol 9:121–126
Iliescu C, Tay FEH, Wei J (2006) Low stress PECVD–SiNx layers at high deposition rates using high power and high frequency for MEMS applications. J Micromech Microeng 16:869–874
Matsuda A (2004) Thin-film silicon-growth process and solar cell application. Jpn J Appl Phys 43:7909–7920
Wu SY, Lee YM, Wu JS, Lin MC (2014) Ab initio chemical kinetics for the unimolecular decomposition of Si2H5 radical and related reverse bimolecular reactions. Int J Quantum Chem 114:278–288
Raghunath P, Lin MC (2013) Ab initio chemical kinetics for SiH2 + Si2H6 and SiH3 + Si2H5 reactions and the related unimolecular decomposition of Si3H8 under a-Si/H CVD conditions. J Phys Chem A 117:10811–10823
Huang WF, Chen HT, Lin MC (2013) Computational investigation of the adsorption and reactions of SiHx (x = 0–4) on TiO2 anatase (101) and rutile (110) surfaces. Int J Quantum Chem 113:1696–1708
Raghunath P, Lee YM, Wu SY, Wu JS, Lin MC (2013) Ab initio chemical kinetics for reactions of H atoms with SiHx (x = 1–3) radicals and related unimolecular decomposition processes. Int J Quantum Chem 113:1735–1746
Raghunath P, Lin MC (2010) Ab initio chemical kinetics for SiH3 reactions with SixH2x+2 (x = 1–4). J Phys Chem A 114:13353–13361
Varma DH, Raghunath P, Lin MC (2010) Ab initio chemical kinetics for the reaction of H atom with Si3H8. J Phys Chem A 114:3642–3648
Wu SY, Raghunath P, Wu JS, Lin MC (2010) Ab initio chemical kinetic study for reactions of H atoms with SiH4 and Si2H6: comparison of theory and experiment. J Phys Chem A 114:633–639
Nguyen TN, Lee YM, Wu JS, Lin MC (2017) Capturing H and H2 by SiH +x (x ≤ 4) ions: comparison between Langevin and quantum statistical models. Jpn J Appl Phys 56:026101
Nguyen TN, Lee YM, Wu JS, Lin MC (2017) Ab initio chemical kinetics for the thermal decomposition of SiH4 + ion and related reverse ion-molecule reactions of interest to PECVD of α-Si: H films. Plasma Chem Plasma Process 37:1249–1264
Nguyen TN, Lin MC (2017) Ab initio chemical kinetics for SiHx reactions with Si2Hy (x = 1, 2, 3, 4; y = 6, 5, 4, 3; x + y = 7) under a-Si: H CVD condition. Int J Chem Kinet 49:197–208
Hess GG, Lampe FW (1966) Ionic reactions in gaseous monosilane. J Chem Phys 44:2257
Henis JMS, Stewart GW, Tripodi MK, Gaspar PP (1972) Ion-molecule reactions in silane. J Chem Phys 57:389
Basner R, Schmidt M, Tarnovsky V, Becker K, Deutsch H (1997) Dissociative ionization of silane by electron impact. Int J Mass Spectrom Ion Process 171:83–93
Cooper G, Ibuki T, Brion CE (1990) Absolute oscillator strengths for photoabsorption, photoionization and ionic photofragmentation of silane. I. The valence shell. Chem Phys 140:133–145
Bleecker DK, Herrebout D, Bogaerts A, Gijbels R, Descamps P (2003) One-dimensional modeling of a capacitively coupled rf plasma in silane/helium, including small concentrations of O2 and N2. J Phys D Appl Phys 36:1826–1833
Kushner MJ (1992) Simulation of the gas phase processes in remote plasma activated chemical vapor deposition of silicon dielectrics using rare gas–silane–ammonia mixtures. J Appl Phys 71:4173
Potzinger P, Larnpe FW (1969) An electron impact study of ionization and dissociation of monosilane and disilane. J Phys Chem 73:3912
Krishnakumar E, Srivastava SK (1995) Ionization cross sections of silane and disilane by electron impact. Contrib Plasma Phys 35:395–404
Pople JA, Curtiss LA (1987) Theoretical thermochemistry. 2. Ionization energies and proton affinities of AHn species (A = C to F and Si to Cl); heats of formation of their cations. J Phys Chem 91:155–162
Berkowitz J, Greene JP, Cho H, Ruščić B (1987) Photoionization mass spectrometric studies of SiHn (n = 1–4). J Chem Phys 86:1235
Börlin K, Heinis T, Jungen M (1986) Photoionization mass spectrometry of silane. Chem Phys 103:93
Ding A, Cassidy RA, Cordis LS, Lampe FW (1985) The photoionization spectra of effusive and supersonic molecular beams of monosilane. J Chem Phys 83:3426
Allen WN, Cheng TMH, Lampe FW (1977) Ion–molecule reactions in SiH4–D2 mixtures. J Chem Phys 66:3371
Boo BH, Armentrout PB (1987) Reaction of silicon ion (2P) with Silane (SiH4, SiD4). Heats of formation of SiHn, SiH +n (n = 1, 2, 3) and Si2H +n (n = 0, 1, 2, 3). Remarkable isotope exchange reaction involving four hydrogen shifts. J Am Chem Soc 109:3549–3559
Boo BH, Armentrout PB (1987) Energetics and reaction mechanisms of reactions of SiH+ + D2, SiD+ + H2, and collision induced dissociation of SiD3 +. J Phys Chem 91:5777–5781
Purvis GD III, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J Chem Phys 76:1910–1918
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157:479–483
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction—a general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Frisch MJ et al (2010) Gaussian 09, revision C.01. Gaussian Inc., Wallingford
Woon DE, Dunning TH (1995) Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J Chem Phys 103:4572
Raghunath P, Nghia NT, Lin MC (2014) Ab initio chemical kinetics of key processes in the hypergolic ignition of hydrazine and nitrogen tetroxide. Adv Quantum Chem 69:253–301
Peterson KA, Woon DE, Dunning TH (1994) Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H + H2 → H2 + H reaction. J Chem Phys 100:7410
Wardlaw DM, Marcus RA (1988) On the statistical theory of unimolecular processes. Adv Chem Phys 70:231
Klippenstein SJ, Wagner AF, Dunbar RC, Wardlaw DM, Robertson SH (1999) VARIFLEX, version 1.00. Argonne National Laboratory, Argonne
Baer T, Hase WL (1996) Unimolecular reactions dynamics: theory and experiment. Oxford University Press, Oxford
Eckart C (1930) The penetration of a potential barrier by electrons. Phys Rev 35:1303–1309
Fernandez-Ramos A, Miller JA, Klippenstein SJ, Truhlar DG (2006) Modeling the kinetics of bimolecular reactions. Chem Rev 106:4518–4584
Paddon-Row MN, Wong SS (1987) On the structure of the SiH4 + cation and its potential energy surface for rearrangement and dissociation: an ab initio M.O. study. J Chem Soc, Chem Commun 4:1585
Wang L, He YL (2008) Halogenated silanes, radicals, and cations: theoretical predictions on ionization energies, structures and potential energy surfaces of cations, proton affinities, and enthalpies of formation. Int J Mass Spectrom 276:56–76
NIST Chemistry WebBook. https://webbook.nist.gov/cgi/cbook.cgi?ID=C31241664&Units=SI&Mask=1000#Diatomic. Accessed 15 Jan 2019
Irikura KK (2007) Experimental vibrational zero-point energies: diatomic molecules. J Phys Chem Ref Data 36:389–397
Cox JD, Wagman DD, Medvedev VA (1989) CODATA key values for thermodynamics. Hemisphere, New York
Berkowitz J, Greene JP, Cho H, Ruščić B (1987) Photoionization mass spectrometric studies of SiHn (n=1–4). J Chem Phys 86:1235
Acknowledgements
This paper (work) is supported by Ministry of Science and Technology, Taiwan (Grant No. MOST107-3017-F009-003) and Ministry of Education, Taiwan (SPROUT Project–Center for Emergent Functional Matter Science of National Chiao Tung University). We also acknowledge the National Center for High-Performing Computers for the use of its facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, T.N., Lee, Y.M., Wu, J.S. et al. Ab Initio Chemical Kinetics for the Thermal Decomposition of SiH2+ and SiH3+ Ions and Related Reverse Ion–Molecule Reactions of Interest to PECVD of α-Si:H Films. Plasma Chem Plasma Process 39, 1559–1573 (2019). https://doi.org/10.1007/s11090-019-10012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10012-x