Abstract
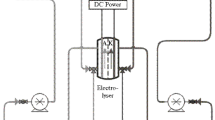
Application of sodium ferrate (Na2FeO4) is considered as the environmental friendly and cost-effective method for oxidation, coagulation and disinfection processes of water and wastewater treatment. Even though many methods of Na2FeO4 production have been proposed, they are still a challenge for the production of large Na2FeO4 quantity. This study aims to verify the optimum operating conditions of Na2FeO4 production by solution plasma process (SPP) of using both anode and cathode made of low-carbon steel placed in electrolyte solution with a distance of 3 cm. The results showed that the efficiency of Na2FeO4 production by SPP that does not obey Faraday’s law is higher than that by conventional electrochemical process. The optimum operating conditions of SPP were verified in 16 M NaOH solution at 30 °C and imposed by 35 V of the voltage while the maximum concentration and the average particle size of the Na2FeO4 production as high as 14.7 mM and 35 nm, respectively, were verified. The maximum current efficiency of 512% and the minimum energy consumption of 6 kWh/kg were verified for the formation of Na2FeO4 during 2 h due to an increased activity of OH− ions. A new approach of large Na2FeO4 production has been proposed to support the development of advanced technologies in water and wastewater treatment.







Similar content being viewed by others
References
Song Y, Men B, Wang D, Ma J (2017) On-line batch production of ferrate with an chemical method and its potential application for greywater recycling with Al(III) salt. J Environ Sci 52:1–7
Zhou Z, Fang S, Chen H, Ji J, Wu J (2017) Trials of treating decentralized domestic sewage from a residential area by potassium ferrate(VI). Water Air Soil Pollut 228:316
Katsoyiannis IA, Tzollas NM, Tolkou AK, Mitrakas M, Ernst M, Zouboulis AI (2017) Use of novel composite coagulants for arsenic removal from waters—experimental insight for the application of polyferric sulfate (PFS). Sustainability 9:590
Banach JL, Sampers I, Van Haute S, van der Fels-Klerx HJ (2015) Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int J Environ Res Public Health 12:8658–8677
Fadavi M, Baboukani AR, Edris H, Salehi M (2018) Study on high-temperature oxidation behaviors of plasma-sprayed TiB2-Co composite coatings. J Korean Ceram Soc 55:178–184
Peiravi M, Mote SR, Mohanty MK, Liu J (2017) Bioelectrochemical treatment of acid mine drainage (AMD) from an abandoned coal mine under aerobic condition. J Hazard Mater 333:329–338
Darvish S, Asadikiya M, Hu B, Singh P, Zhong Y (2016) Thermodynamic prediction of the effect of CO2 to the stability of (La0.8Sr0.2)0.98MnO3±δ system. Int J Hydr Energy 41:10239–10248
Liu J, Weinholtz L, Zheng L, Peiravi M, Wu Y, Chen D (2017) Removal of PFOA in groundwater by Fe0 and MnO2 nanoparticles under visible light. J Environ Sci Health A Toxic/Hazard Subst Environ Eng 52:1048–1054
Talaiekhozani A, Salari M, Talaei MR, Bagheri M, Eskandari Z (2016) Formaldehyde removal from wastewater and air by using UV, ferrate(VI) and UV/ferrate(VI). J Environ Manag 184:204–209
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30:11–26
Petrov VG, Perfiliev YD, Dedushenko SK, Kuchinskaya TS, Kalmykov SN (2016) Radionuclide removal from aqueous solutions using potassium ferrate(VI). J Radioanal Nucl Chem 310:347–352
Wang C, Klamerth N, Huang R, Elnakar H, Gamal El-Din M (2016) Oxidation of oil sands process-affected water by potassium ferrate(VI). Environ Sci Technol 50:4238–4247
Goodwill JE, Jiang Y, Reckhow DA, Gikonyo J, Tobiason JE (2015) Characterization of particles from ferrate preoxidation. Environ Sci Technol 49:4955–4962
Lv D, Zheng L, Zhang H, Deng Y (2018) Coagulation of colloidal particles with ferrate(VI). Environ Sci Water Res Technol 4:701–710
Zheng L, Deng Y (2016) Settleability and characteristics of ferrate(VI)-induced particles in advanced wastewater treatment. Water Res 93:172–178
Saebnoori E, Navidinejad A, Baboukani AR (2016) Mechanism study and parameter optimization of A356 aluminum alloy electrochemical polishing. In: Proceedings of Iran international aluminum conference (IIAC2016), 11–12 May 2016, Tehran, IR Iran
Talaiekhozani A, Talaei MR, Rezania S (2017) An overview on production and application of ferrate(VI) for chemical oxidation, coagulation and disinfection of water and wastewater. J Environ Chem Eng 5:1828–1842
Schmidbaur H (2018) The history and the current revival of the oxo chemistry of iron in its highest oxidation states: FeVI–FeVIII. J Inorg Gen Chem 644:536–559
Krajewski M, Brzozka K, Lin WS, Lin HM, Tokarczyk M, Borysiuk J, Kowalski G, Wasik D (2016) High temperature oxidation of iron–iron oxide core–shell nanowires composed of iron nanoparticles. Phys Chem Chem Phys 18:3900–3909
Pujilaksono B, Jonsson T, Halvarsson M, Svensson JE, Johansson LG (2010) Oxidation of iron at 400–600 °C in dry and wet O2. Corros Sci 52:1560–1569
Wei Y-L, Wang Y-S, Liu C-H (2015) Preparation of potassium ferrate from spent steel pickling liquid. Metals 5:1770–1787
Schmidbaur H (2018) The history and the current revival of the oxo chemistry of iron in its highest oxidation states: FeVI–FeVIII. Z Anorg Allg Chem 644:536–559
De Koninck M, Brousse T, Bélanger D (2003) The electrochemical generation of ferrate at pressed iron powder electrodes: effect of various operating parameters. Electrochim Acta 48:1425–1433
He W, Wang J, Yang C, Zhang J (2006) The rapid electrochemical preparation of dissolved ferrate(VI): effects of various operating parameters. Electrochim Acta 51:1967–1973
Darvish S, Zhong Y, Gopalan S (2017) Thermodynamic stability of La0.6Sr0.4Co0.2Fe0.8O3−δ in carbon dioxide impurity: a comprehensive experimental and computational assessment. ECS Trans 78:1021–1025
Wang CC, He S, Chen K, Rowles MR, Darvish S, Zhong Y, Jiang SP (2017) Effect of SO2 poisoning on the electrochemical activity of La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes of solid oxide fuel cells. J Electrochem Soc 164:F514–F524
Kareem TA, Kaliani AA (2012) Glow discharge plasma electrolysis for nanoparticles synthesis. Ionics 18:315–327
Hyun K, Saito N (2017) The solution plasma process for heteroatom-carbon nanosheets: the role of precursors. Sci Rep 7:3825
Pootawang P, Saito N, Lee SY (2013) Discharge time dependence of a solution plasma process for colloidal copper nanoparticle synthesis and particle characteristics. Nanotechnol 24:055604
Saito G, Hosokai S, Tsubota M, Akiyama T (2011) Nickel nanoparticles formation from solution plasma using edge-shielded electrode. Plasma Chem Plasma Process 31:719–728
Saito G, Hosokai S, Tsubota M, Akiyama T (2012) Influence of solution temperature and surfactants on morphologies of tin oxide produced using a solution plasma technique. Cryst Growth Des 12:2455–2459
Saito G, Hosokai S, Tsubota M, Akiyama T (2011) Synthesis of copper/copper oxide nanoparticles by solution plasma. J Appl Phys 110:023302
Ding L, Liu T-X, Li X-Z (2014) Removal of CH3SH with in situ generated ferrate(VI) in a wet-scrubbing reactor. J Chem Technol Biotechnol 89:455–461
Alsheyab M, Jiang J-Q, Stanford C (2010) Electrochemical generation of ferrate(VI): determination of optimum conditions. Desalination 254:175–178
Barışçı S, Ulu F, Sarkka H, Dimoglo A, Sillanpaa M (2014) Electrosynthesis of ferrate(VI) ion using high purity iron electrodes: optimization of influencing parameters on the process and investigating its stability. Int J Electrochem Sci 9:3099–3117
Wang H, Liu Y, Zeng F, Song S (2015) Electrochemical synthesis of ferrate(VI) by regular anodic replacement. Int J Electrochem Sci 10:7966–7976
Gao J, Ma D, Lu Q, Li Y, Li X, Yang W (2010) Synthesis and characterization of montmorillonite-graft-acrylic acid superabsorbent by using glow-discharge electrolysis plasma. Plasma Chem Plasma Process 30:873–883
Le Formal F, Tétreault N, Cornuz M, Moehl T, Grätzel M, Sivula K (2011) Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem Sci 2:737–743
Hull CC, Crofts NC (1996) Determination of the total attenuation coefficient for six contact lens materials using the Beer–Lambert law. Ophthal Physiol Opt 16:150–157
Sivasubramanian H (2018) Effect of ignition delay (ID) on performance, emission and combustion characteristics of 2-methyl furan-unleaded gasoline blends in a MPFI SI engine. Alex Eng J 57:499–507
Yu X, Licht S (2008) Advances in electrochemical Fe(VI) synthesis and analysis. J Appl Electrochem 38:731–742
Hassannejad H, Moghaddasi M, Saebnoori E, Baboukani AR (2017) Microstructure, deposition mechanism and corrosion behavior of nanostructured cerium oxide conversion coating modified with chitosan on AA2024 aluminum alloy. J Alloys Compd 725:968–975
Ding L (2013) Removal of methyl mercaptan from foul gas by in situ production of ferrate(VI) for odour control. Ph.D. Thesis, The Hong Kong Polytechnic University
Sharma VK, Chen L, Zboril R (2016) Review on high valent FeVI (Ferrate): a sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain Chem Eng 4:18–34
Kim SC, Kim SM, Yoon GJ, Nam SW, Lee SY, Kim JW (2014) Gelatin-based sponge with Ag nanoparticles prepared by solution plasma: fabrication, characteristics, and their bactericidal effect. Curr Appl Phys 14:S172–S179
Ren J, Yao M, Yang W, Li Y, Gao J (2014) Recent progress in the application of glow-discharge electrolysis plasma. Cent Eur J Chem 12:1213–1221
Bouzek K, Lipovská M, Schmidt M, RoušarDeceased I, Wragg AA (1998) Electrochemical production of ferrate(VI) using sinusoidal alternating current superimposed on direct current: grey and white cast iron electrodes. Electrochim Acta 44:547–557 (This paper is dedicated to the memory of Professor Ivo Roušar)
Fletcher S (2009) Tafel slopes from first principles. J Solid State Electrochem 13:537–549
Zou J-Y, Chin D-T (1987) Mechanism of steel corrosion in concentrated NaOH solutions. Electrochim Acta 32:1751–1756
Tiwari D, Lee S-M (2011) Ferrate(VI) in the treatment of wastewaters: a new generation green chemical. In: García Einschlag FS (ed) Wastewater—treatment reutilization. INTECH, London, pp 241–276
Sun X, Zu K, Liang H, Sun L, Zhang L, Wang C, Sharma VK (2018) Electrochemical synthesis of ferrate(VI) using sponge iron anode and oxidative transformations of antibiotic and pesticide. J Hazard Mater 344:155–1164
Fulazzaky MA, Ali N, Samekto H, Ghazali MI (2012) Assessment of CpTi surface properties after nitrogen ion implantation with various doses and energies. Metall Mater Trans A 43:4185–4193
Baboukani AR, Adelowo E, Agrawal R, Khakpour I, Drozd V, Li W, Wang C (2018) Electrostatic spray deposited Sn-SnO2-CNF composite anodes for lithium ion storage. ECS Trans 85:331–336
Manoli K, Nakhla G, Feng M, Sharma VK, Ray AK (2017) Silica gel-enhanced oxidation of caffeine by ferrate(VI). Chem Eng J 330:987–994
Foroughi P, Rabiei Baboukani A, Franco Hernandez A, Wang C, Cheng Z (2018) Phase control during synthesis of nanocrystalline ultrahigh temperature tantalum-hafnium diboride powders. J Am Ceram Soc 101:5745–5755
Zolfaghari S, Baboukani AR, Ashrafi A, Saatchi A (2018) Investigation the effects of Na2MoO4 as an inhibitor on electrochemical corrosion behavior of 316L stainless steel in LiBr solution. Zaštita Materijala 59:108–116
Saberi F, Boroujeny BS, Doostmohamdi A, Baboukani AR, Asadikiya M (2018) Electrophoretic deposition kinetics and properties of ZrO2 nano coatings. Mater Chem Phys 213:444–454
Saito G, Hosokai S, Akiyama T (2011) Synthesis of ZnO nanoflowers by solution plasma. Mater Chem Phys 130:79–83
Sharma VK, Tolan S, Bumbálek V, Macova Z, Bouzek K (2016) Stability of ferrate(VI) in 14 M NaOH–KOH mixtures at different temperatures. ACS Symp Ser 1238:241–253
Sun X, Zhang Q, Liang H, Ying L, Xiangxu M, Sharma VK (2016) Ferrate(VI) as a greener oxidant: electrochemical generation and treatment of phenol. J Hazard Mater 319:130–136
Tan W-F, Yu Y-T, Wang M-X, Liu F, Koopal LK (2014) Shape evolution synthesis of monodisperse spherical, ellipsoidal, and elongated hematite (α-Fe2O3) nanoparticles using ascorbic acid. Cryst Growth Des 14(157):164
Wu W, Wu Z, Yu T, Jiang C, Kim WS (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16:023501
El Maghraoui A, Zerouale A, Ijjaali M, Sajieddine M (2013) Synthesis and characterization of ferrate(VI) alkali metal by electrochemical method. Adv Mater Phys Chem 3:83–87
Lescuras-Darrou V, Lapicque F, Valentin G (2002) Electrochemical ferrate generation for waste water treatment using cast irons with high silicon contents. J Appl Electrochem 32:57–63
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samimi-Sedeh, S., Saebnoori, E., Talaiekhozani, A. et al. Assessing the Efficiency of Sodium Ferrate Production by Solution Plasma Process. Plasma Chem Plasma Process 39, 769–786 (2019). https://doi.org/10.1007/s11090-019-09989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09989-2