Abstract
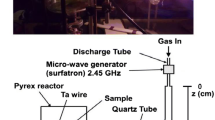
We report a detailed comparison between RF and microwave (HF) plasmas of N2 and Ar–20 %N2 as well as in the corresponding afterglows by comparing densities of active species at nearly the same discharge conditions of tube diameter (5–6 mm), gas pressure (6–8 Torr), flow rate (0.6–1.0 slm) and applied power (50–150 W). The analysis reveals an interesting difference between the two cases; the length of the RF plasma (~25 cm) is measured to be much longer than that of HF (6 cm). This ensures a much longer residence time (10−2 s) of the active species in the N2 RF plasma [compared to that (10−3 s) of HF], providing a condition for an efficient vibrational excitation of N2(X, v) by (V–V) climbing-up processes, making the RF plasma more vibrationally excited than the HF one. As a result of high V–V plasma excitation in RF, the densities of the vibrationally excited N2(X, v > 13) molecules are higher in the RF afterglow than in the HF afterglow. Destruction of N2(X, v) due to the tube wall is estimated to be very similar between the two system as can be inferred from the γv destruction probability of N2(X, v > 3–13) on the tube wall (2–3 × 10−3 for both cases) obtained from a comparison between the density of N2(X, v > 3–9) in the plasmas to that of the N2(X, v > 13) in the long afterglows. Interestingly enough, densities of N-atoms and N2(A) metastable molecules in the afterglow regions, however, are measured to be very similar with each other. The measured lower density of N2 + ions than expected in the HF afterglow is rationalized from a high oxygen impurity in our HF setup since N2 + ions are very sensitive to oxygen impurity .


Similar content being viewed by others
References
Ricard A, Oh SG, Guerra V (2013) Line-ratio determination of atomic oxygen and N2 metastable absolute densities in an RF nitrogen late afterglow. Plasma Sources Sci Technol 22(3):035009. doi:10.1088/0963-0252/22/3/035009
Zerrouki H, Ricard A, Sarrette JP (2013) Determination of N and O-atom and N2(A) metastable molecule densities in the afterglows of N2 and N2–O2 microwave discharges. Contrib Plasmas Phys 53(8):599–604. doi:10.1002/ctpp.201300008
Ricard A, Oh SG (2014) Densities of active species in N2 and N2–H2 RF pink afterglow. Plasma Sources Sci Technol 23(4):045009. doi:10.1088/0963-0252/23/4/045009
Zerrouki H, Ricard A, Sarrette JP (2014) Determination of N and O-atoms and N2(A) metastable molecule densities in the afterglows of N2–H2, Ar–N2–H2 and Ar–N2–O2 microwave discharges. Contrib Plasmas Phys 54(10):827–837. doi:10.1002/ctpp.201400001
Ricard A, Oh SG, Jang J, Kim YK (2015) Quantitative evaluation of the densities of active species of N2 in the afterglow of Ar-embedded N2 RF plasma. Curr Appl Phys 15(11):1453–1462. doi:10.1016/j.cap.2015.08.013
Philip N, Saoudi B, Crevier MC, Moisan M, Barbeau J, Pelletier J (2002) The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: the case of N2–O2 mixtures. IEEE Trans Plasma Sci 30(4):1429–1436. doi:10.1109/TPS.2002.804203
Villeger S, Sarrette JP, Ricard A (2005) Synergy between N and O atom action and substrate surface temperature in a sterilization process using a flowing N2–O2 microwave post discharge. Plasma Process Polym 2(9):709–714. doi:10.1002/ppap.200500040
Pointu A-M, Ricard A, Dodet B, Odic E, Larbre J, Ganciu M (2005) Production of active species in N2–O2 flowing post-discharges at atmospheric pressure for sterilization. J Phys D Appl Phys 38(12):1905–1909. doi:10.1088/0022-3727/38/12/009
Ricard A, Jaoul C, Gaboriau F, Gherardi N, Villeger S (2004) Production of N, H, O, and C atoms in flowing microwave discharges. Surf Coat Technol 188–189:287–293. doi:10.1016/j.surfcoat.2004.08.171
Ricard A, Monna V (2002) Reactive molecular plasmas. Plasma Sources Sci Technol 11(3A):A150. doi:10.1088/0963-0252/11/3A/322
Molchan IS, Thompson GE, Skeldon P, Trigoulet N, Chapon P, Tempez A, Malherbe J, Lobo Revilla L, Bordel N, Belenguer P, Nelis T, Zahri A, Therese L, Guillot P, Ganciu M, Michler J, Hohl M (2009) The concept of plasma cleaning in glow discharge spectrometry. J Anal At Spectrom 24(6):734–741. doi:10.1039/B818343K
Kaluri SR, Hess DW (1996) Nitrogen incorporation in thin oxides by constant current N2O plasma anodization of silicon and N2 plasma nitridation of silicon oxides. Appl Phys Lett 69(8):1053–1055. doi:10.1063/1.116928
Ricard A, Hubert J, Michel H (1992) Correlations between active plasma species and steel surface nitriding in microwave post-discharge reactors. In: Capitelli M, Gorse C (eds) Plasma technology: fundamentals and applications. Springer, Boston, pp 125–142. doi:10.1007/978-1-4615-3400-6_9
Ricard A, Czerwiec T, Belmonte T, Bockel S, Michel H (1999) Detection by emission spectroscopy of active species in plasma–surface processes. Thin Solid Films 341(1–2):1–8. doi:10.1016/S0040-6090(98)01529-6
Zhu X-M, Pu Y-K (2010) Optical emission spectroscopy in low-temperature plasmas containing argon and nitrogen: determination of the electron temperature and density by the line-ratio method. J Phys D Appl Phys 43(40):403001. doi:10.1088/0022-3727/43/40/403001
Zerrouki H, Ricard A, Sarrette JP (2014) Determination of N and O-atoms, of N2 (A) and N2 (X, v > 13) metastable molecules and N2+ ion densities in the afterglows of N2–H2, Ar–N2–H2 and Ar–N2–O2 microwave discharges. J Phys Conf Ser 550(1):012045. doi:10.1088/1742-6596/550/1/012045
Kang N, Lee M, Ricard A, S-g Oh (2012) Effect of controlled O2 impurities on N2 afterglows of RF discharges. Curr Appl Phys 12(6):1448–1453. doi:10.1016/j.cap.2012.04.009
Boudam MK, Saoudi B, Moisan M, Ricard A (2007) Characterization of the flowing afterglows of an N2–O2 reduced-pressure discharge: setting the operating conditions to achieve a dominant late afterglow and correlating the NO β UV intensity variation with the N and O atom densities. J Phys D Appl Phys 40(6):1694–1711. doi:10.1088/0022-3727/40/6/019
Britun N, Gaillard M, Ricard A, Kim YM, Kim KS, Han JG (2007) Determination of the vibrational, rotational and electron temperatures in N2 and Ar–N2 RF discharge. J Phys D Appl Phys 40(4):1022. doi:10.1088/0022-3727/40/4/016
Nassar H, Pellerin S, Musiol K, Martinie O, Pellerin N, Cormier JM (2004) N2 +/N2 ratio and temperature measurements based on the first negative N2 + and second positive N2 overlapped molecular emission spectra. J Phys D Appl Phys 37(14):1904–1916. doi:10.1088/0022-3727/37/14/005
Sadeghi N, Foissac C, Supiot P (2001) Kinetics of N2 (A 3Σ +u ) molecules and ionization mechanisms in the afterglow of a flowing N2 microwave discharge. J Phys D Appl Phys 34(12):1779. doi:10.1088/0022-3727/34/12/304
Chaker M, Moisan M, Zakrzewski Z (1986) Microwave and RF surface wave sustained discharges as plasma sources for plasma chemistry and plasma processing. Plasma Chem Plasma Process 6:79–96
Massabieaux B, Plain A, Ricard A, Capitelli M, Gorse C (1983) Excitation of vibrational and electronic states in a glow discharge column in flowing N2. J Phys B At Mol Phys 16(10):1863. doi:10.1088/0022-3700/16/10/021
Gordiets B, Hamedov SS, Shelepin IA (1975) Vibrational kinetics of harmonic oscillators under essentially nonequilibrium conditions. Sov Phys JETP 40:640
Gilmore FR, Laher RR, Espy PJ (1992) Franck–Condon factors, r-centroids, electronic transition moments, and Einstein coefficients for many nitrogen and oxygen band systems. J Phys Chem Ref Data 21(5):1005–1107. doi:10.1063/1.555910
Pancheshnyi SV, Starikovskaia SM, Starikovskii AY (2000) Collisional deactivation of N2(C 3Πu, v = 0, 1, 2, 3) states by N2, O2, H2 and H2O molecules. Chem Phys 262(2–3):349–357. doi:10.1016/S0301-0104(00)00338-4
De Benedictis S, Dilecce G (1995) Vibrational relaxation of N2(C, v) state in N2 pulsed RF discharge: electron impact and pooling reactions. Chem Phys 192(2):149–162. doi:10.1016/0301-0104(94)00370-P
Mavadat M, Ricard A, Sarra-Bournet C, Laroche G (2011) Determination of ro-vibrational excitations of N2 (B, v′) and N2 (C, v′) states in N2 microwave discharges using visible and IR spectroscopy. J Phys D Appl Phys 44(15):155207. doi:10.1088/0022-3727/44/15/155207
Blanchard H, Sarrette JP, Villeger S, Baudel P, Ricard A (2004) Density of oxygen atoms in high pressure (10–50 torr) flowing microwave post-discharges for elastomer treatments. Eur Phys J Appl Phys 26(03):221–226. doi:10.1051/epjap:2004036
Capitelli M, Gorse C, Ricard A (1982) Non equilibrium dissociation and ionization of N2 in decaying plasmas. J Phys (Paris) 43:L417–L423
Villeger S, Sarrette JP, Rouffet B, Cousty S, Ricard A (2008) Treatment of flat and hollow substrates by a pure nitrogen flowing post discharge. Eur Phys J Appl Phys 42(01):25–32. doi:10.1051/epjap:2007177
Marinov D, Lopatik D, Guaitella O, Hubner M, Ionikh Y, Ropcke J, Rousseau A (2012) Surface vibrational relaxation of N2 studied by CO2 titration with time-resolved quantum cascade laser absorption spectroscopy. J Phys D Appl Phys 45(175201):33
Guerra V, Loureiro J (1997) Self-consistent electron and heavy particle kinetics in a low-pressure N2–O2 glow discharge. Plasmas Sources Sci Technol 6:373–385
Armenise I, Capitelli M, Garcia E, Gorse C, Lagana A, Longo S (1992) Deactivation dynamics of vibrationally excited nitrogen molecules by nitrogen atoms. Chem Phys Lett 200:597–604
Capitelli M, Gorse C, Ricard A (1986) Coupling of vibrational and electronic energy distributions in discharge and post-discharge conditions. In: Topics in current chemistry, vol 39. Non equilibrium vibrational kinetics. Chapter 11, pp 315–337
Acknowledgments
Y.K.K. acknowledges a financial support from the International Research and Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015K1A3A1A21000248). This work was supported by the Franco-Korean project PHC STAR 2015 (34306TK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricard, A., Sarrette, JP., Oh, SG. et al. Comparison of the Active Species in the RF and Microwave Flowing Discharges of N2 and Ar–20 %N2 . Plasma Chem Plasma Process 36, 1559–1570 (2016). https://doi.org/10.1007/s11090-016-9739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9739-9