Abstract
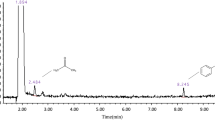
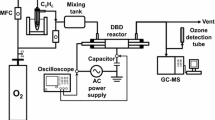
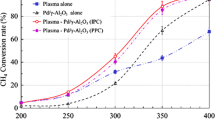
The performance of NiO, MnO2, CeO2, Fe2O3, and CuO catalysts on alumina in removing toluene from a gas stream was studied in a plasma catalysis system. The NiO catalyst performed better than the other catalysts, generating more toluene-destroying oxygen species by decomposing ozone. The optimum nickel loading in the NiO/γ-Al2O3 catalyst was approximately 5 wt%, close to the monolayer dispersion threshold of NiO on γ-Al2O3. The presence of water vapor had a negative effect on catalytic performance due to its quenching of high speed electrons and its competition with toluene for adsorption sites. Water vapor also reduced the outlet ozone concentration by inhibiting the production of key intermediate in the ozone formation process.










Similar content being viewed by others
References
Zheng JY, Shao M, Che WW, Zhang LJ, Zhong LJ, Zhang YH, Streets D (2009) Environ Sci Technol 43:8580–8586
Shi J, Zhao ZX, Xia QB, Li YW, Li Z (2011) J Chem Eng Data 56:3419–3425
Kim HH (2004) Plasma Process Polym 1:91–110
Schiorlin M, Marotta E, Rea M, Paradisi C (2009) Environ Sci Technol 43:9386–9392
Mista W, Kacprzyk R (2008) Catal Today 137:345–349
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Environ Sci Technol 43:2216–2227
Van Durme J, Dewulf J, Leys C, Van Langenhove H (2008) Appl Catal B Environ 78:324–333
Vandenbroucke AM, Morent R, De Geyter N, Leys C (2011) J Hazard Mater 195:30–54
Fan X, Zhu TL, Sun YF, Yan X (2011) J Hazard Mater 196:380–385
Karuppiah J, Karvembu R, Subrahmanya Ch (2012) Chem Eng J 180:39–45
Stoyanova M, Konova P, Nikolov P, Naydenov A, Christoskova St, Mehandjiev D (2006) Chem Eng J 122:41–46
Kim HH, Ogata A, Futamura S (2006) IEEE Trans Plasma Sci 34:984–995
Ogata A, Einaga H, Kabashima H, Futamura S, Kushiyama S, Kim HH (2003) Appl Catal B Environ 46:87–95
Wang XY, Zhao BY, Jiang DE, Xie YC (1999) Appl Catal A Gen 188:201–209
Pan HY, Xu MY, Li Z, Huang SS, He C (2009) Chemosphere 76:721–726
Zhao ZX, Li XM, Huang SS, Xia QB, Li Z (2011) Ind Eng Chem Res 50:2254–2261
Zhang ZJ, Xian SK, Xi HX, Wang HH, Li Z (2011) Chem Eng Sci 66:4878–4888
Harling AM, Glover DJ, Whitehead JC, Zhang K (2009) Appl Catal B Environ 90:157–161
NIST, Chemical Kinetics Database on the Web Standard Reference Database 17, Version 7.0 (Web Version), Release 1.6.5, 2012 http://kinetics.nist.gov/kinetics/
Chavadej S, Kiatubolpaiboon W, Rangsunvigit P, Sreethawong T (2007) J Mol Catal A Chem 263:128–136
Magureanu M, Mandache NB, Eloy P, Gaigneaux EM, Parvulescu VI (2005) Appl Catal B Environ 61:12–20
Futamura S, Einaga H, Kabashima H, Hwan LY (2004) Catal Today 89:89–95
Molina R, Poncelet G (1998) J Catal 173:257–267
Akande AJ, Idem RO, Dalai AK (2005) Appl Catal A Gen 287:159–175
Yu XX, Wu NZ, Xie YC, Tang YQ (2000) J Mater Chem 10:1629–1634
Ogata A, Shintani N, Yamanouchi K, Mizuno K, Kushiyama S, Yamamoto T (2000) Plasma Chem Plasma P 20:453–467
Huang HB, Ye DQ, Leung Dennis YC (2011) IEEE T Plasma Sci 39:576–580
Fan X, Zhu TL, Wan YJ, Xiao X (2010) J Hazard Mater 180:616–621
Huang HB, Ye DQ, Leung Dennis YC, Feng FD, Guan XJ (2011) J Mol Catal A Chem 336:87–93
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 20936001, U1201231), the Science Foundation of Guangdong Province, and the State Key Lab of Subtropical Building Science, South China University of Technology (Grant C710090Z). We are grateful to Dr. Donald George Barnes for providing helpful advice to improve our paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Huang, Y., Xia, Q. et al. Decomposition of Toluene in a Plasma Catalysis System with NiO, MnO2, CeO2, Fe2O3, and CuO Catalysts. Plasma Chem Plasma Process 33, 1073–1082 (2013). https://doi.org/10.1007/s11090-013-9485-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9485-1