Abstract
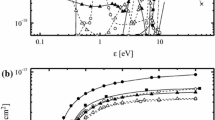
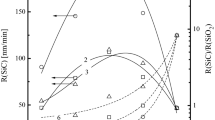
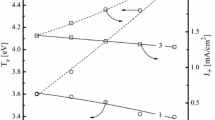
The effects of HBr/Ar and HBr/Cl2 mixing ratios in the ranges of 0–100% Ar or Cl2 on plasma parameters, densities of active species influencing the dry etch mechanisms were analyzed at fixed total gas flow rate of 40 sccm, total gas pressure of 6 mTorr, input power of 700 W and bias power of 300 W. The investigation combined plasma diagnostics by Langmuir probes and the 0-dimensional plasma modeling. It was found that the dilution of HBr by Ar results in maximum effect on the ion energy flux with expected impact on the etch rate in the ion-flux-limited etch regime, while the addition of Cl2 influences mainly the relative fluxes of Br and Cl atoms on the etched surface with expected impact on the etch rate in the reaction-rate-limited etch regime.





Similar content being viewed by others
References
Pearton SJ, Chakrabarti UK, Lane E, Perley AP, Abernathy CR, Hobson WS, Jones KS (1992) J Electrochem Soc 139:856
Kuo Y, Tai TL (1998) J Electrochem Soc 145:4313
Bestwick TD, Oehrlane GS (1990) J Vac Sci Technol A 8:1696
Jin W, Vitale SA, Sawin HH (2002) J Vac Sci Technol A 20:2106
Bazin A, Pargon E, Mellhaoui X, Perret D, Mortini B, Joubert O (2008) Advances in resist materials and processing technology XXV. In: Henderson CL (ed) Proceedings of the SPIE. 6923, p 692337
Pargon E, Menguelti K, Martin M, Bazin A, Chaix-Pluchery O, Sourd C, Derrough S, Lill T, Joubert O (2009) J Appl Phys 105:094902
Lee C, Lieberman MA (1995) J Vac Sci Technol A 13:368
Ashida S, Lieberman MA (1997) Jpn J Appl Phys 36:854
Lieberman MA, Ashida S (1996) Plasma Sources Sci Technol 5:145
Efremov A, Choi B-G, Nahm S, Lee HW, Min N-K, Kwon K-H (2008) J Korean Phys Soc 52:48
Lee HW, Kim M, Min N-K, Efremov A, Lee C-W, Kwon K-H, Jpn J (2008) Appl Phys 47:6917
Kim M, Min N-K, Yun SJ, Lee HW, Efremov A, Kwon K-H (2008) Microelectron Eng 85:348
Johnson EO, Malter L (1950) Phys Rev 80:58
Sugavara M (1998) Plasma etching. Fundamentals and applications. Oxford University Press Inc., New York
Ullal SJ, Godfrey AR, Edelberg E, Braly L, Vahedy V, Aydil ES (2002) J Vac Sci Technol A 20:43
Malyshev MV, Donnelly VM (2000) J Appl Phys 87:1642
Hopwood J, Guarnieri CR, Whitehair SJ, Cuomo JJ (1993) J Vac Sci Technol A 11:152
Efremov AM, Kim G-H, Kim J-G, Kim C-I (2007) Thin Solid Films 515:5395
Šašić O, Dujko S, Petrović Z (2007) Jpn J Appl Phys 46:3560
Kurepa MV, Babic DS, Belic DS (1981) J Phys B At Mol Phys 14:375
Gudmundsson JT (2001) Plasma Sources Sci Technol 10:76
Morgan WL (1992) Plasma Chem Plasma Proc 12:449
NIST chemical kinetica database. (2010) http://kinetics.nist.gov/kinetics/
Chantry PJ (1987) J Appl Phys 62:1141
Lieberman MA, Lichtenberg AJ (1994) Principles of plasma discharges and materials processing. Wiley, New York
Dzotsenidze Z, Petviashvili D, Museridze M, Sulaberidze K (2001) Bull Ga Acad Sci 164
Serdyuk NK, Gutorov VV, Panfilov VN (1981) React Kinet Catal Lett 16:393
Efremov A, Min N-K, Choi B-G, Baek K-H, Kwon K-H (2008) J Electrochem Soc 155:D777
Corr CS, Despiau-Pujo E, Chabert P, Graham WG, Marro FG, Graves DB (2008) J Phys D Appl Phys 41:185202
Curley GA, Gatilova L, Guilet S, Bouchoule S, Gogna GS, Sirse N, Karkari S, Booth JP (2010) J Vac Sci Technol. A 28:360
Kota GP, Coburn JW, Graves DB (1998) J Vac Sci Technol A 16:270
Wood BJ, Wise H (1961) J Phys Chem 65:1976
Efremov AM, Kim GH, Balashov DI, Kim C-I (2006) Vacuum 81:244
Efremov AM, Kim GH, Kim JG, Bogomolov AV, Kim CI (2007) Microelectron Eng 84:136
Fuller NCM, Donnelly VM, Herman IP (2002) J Vac Sci Technol A 20:170
Fuller NCM, Herman IP, Donnelly VM (2001) J Appl Phys 90:3182
Gray DC, Tepermeister I, Sawin HH (1993) J Vac Sci Technol B 11:1243
Winters HW, Coburn JW (1992) Surf Sci Rep 14:162
Lee C, Graves DB, Lieberman MA (1996) Plasma Chem Plasma Process 16:99
Efremov AM, Kim DP, Kim C-I (2004) IEEE Trans Plasma Sci 32:1344
Acknowledgment
This work was supported by a Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Efremov, A., Kim, Y., Lee, HW. et al. A Comparative Study of HBr-Ar and HBr-Cl2 Plasma Chemistries for Dry Etch Applications. Plasma Chem Plasma Process 31, 259–271 (2011). https://doi.org/10.1007/s11090-010-9279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9279-7