Abstract
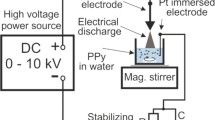
An approach for polymerization to produce polymethylmethacrylate (PMMA) was developed, in which the reaction was initiated by the glow discharge electrolysis (GDE) rather than chemical initiators. The highest number-average molecular weight (M n) and the lowest polydispersity index (PDI) of the resulting polymer were 1.12 × 106 g/mol and 1.21, respectively. The following parameters such as the applied voltage, discharge time, the content of methylmethacrylate (MMA), the amount of a suspension stabilizer (polyvinyl alcohol), polymerization temperature and time were examined in detail, which could affect the conversion, molecular weight and polydispersity index. The M n and PDI of polymer can be monitored by changing the discharge parameters and polymerization conditions. PMMA was characterized by gel permeation chromatography (GPC), Fourier transform infrared spectroscopy (FT-IR), cold field emission scanning electron microscopy (FESEM), nuclear magnetic resonance (NMR) and thermogravimetric analysis (TGA). Results indicate that using the GDE technique to initiate the polymerization reaction is successful, because the product obtained has the same properties with one obtained by chemical method, for example, in chemical structure, tacticity and thermal stability. Moreover, the polymer particles for the former are smaller than the latter. The kinetic observation was that the polymerization of MMA initiated by the GDE plasma obeys the first order of reaction with an obvious induction period.













Similar content being viewed by others
References
Zhou XD, Ni PH, Yu ZQ (2007) Polymer 48:6262–6271
Zhou Y, Zhu J, Zhu XL, Cheng ZP (2006) Radiat Phys Chem 75:485–492
Teo BM, Prescott SW, Ashokkumar M, Grieser F (2008) Ultrason Sonochem 15:89–94
Zhang ZB, Zhu XL, Zhu J, Cheng ZP (2006) Polym Bull 56:539–548
Filardo G, Caputo G, Galia A, Calderaro E, Spadaro G (2000) Macromolecules 33:278–283
Gu CB, Wang DJ, Wang XQ, Huang Y, Zhen Z, Liu XH (2002) J Appl Polym Sci 86:1731–1735
Chen GJ, Zhu XL, Zhu J, Cheng ZP (2004) Macromol Rapid Commun 25:818–824
Joshi R, Schulze RD, Meyer-Plath A, Friedrich JF (2008) Plasma Process Polym 5:695–707
Blanc D, Last A, Franc J, Pavan S, Loubet JL (2006) Thin Solid Films 515:942–946
Tanglumlert W, Prasassarakich P, Supaphol P, Wongkasemjit S (2006) Surf Coat Tech 200:2784–2790
Guo YB, Hong FC-N (2003) Diam Relat Mater 12:946–952
Chau JLH, Hsieh C-C, Lin Y-M, Li A-K (2008) Prog Org Coat 62:436–439
Sengupta SK, Singh R, Srivastava AK (1998) J Electrochem Soc 145:2209–2213
Gao JZ, Wang AX, Fu Y, Wu JL, Ma DP, Guo X, Li Y, Yang W (2008) Plasma Sci Technol 10:30–38
Denaro AR, Hickling A (1958) J Electrochem Soc 105:265–270
Hickling A, Ingram MD (1964) J Electroanal Chem 8:65–81
Sengupta SK, Singh R, Srivastava AK (1998) Indian J Chem 37:558–560
Sengupta SK, Sandhir U, Misra N (2001) J Polym Sci Part A Polym Chem 39:1584–1588
Gao JZ, Wang AX, Li Y, Fu Y, Wu JL, Wang YD, Wang YJ (2008) React Funct Polym 68:1377–1383
Zhang ZB, Zhu XL, Zhu J, Cheng ZP (2006) Polymer 47:6970–6977
Acknowledgments
This work was supported in part by the Key Project of Science and Technology of Education Ministry (00250), the Natural Science Foundation of Gansu Province (3ZS041-A25-028), the Project of KJCXGC-01, NWNU, and Gansu Key Lab of Polymer Materials, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, A., Gao, J., Yuan, L. et al. Synthesis and Characterization of Polymethylmethacrylate by Using Glow Discharge Electrolysis Plasma. Plasma Chem Plasma Process 29, 387–398 (2009). https://doi.org/10.1007/s11090-009-9185-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-009-9185-z