Abstract
Model alloys, Fe–20Cr and Fe–20Cr–20Ni (wt%) with and without 1% Si, were exposed to four different gas mixtures Ar–10H2O, Ar–10H2O–0.1HCl, Ar–10H2O–0.1HCl–10CO2, Ar–10H2O–0.1HCl–10CO2–0.5SO2 (vol.%) at 650 °C. The Fe–20Cr and Fe–20Cr–20Ni alloys underwent breakaway corrosion in Ar–10H2O–(0.1HCl)–(10CO2), forming Cr2O3 and Fe-rich oxide nodules. Silicon addition markedly increased corrosion resistance for both alloys by forming an additional SiO2 layer beneath the Cr2O3 scale. Addition of SO2 to Ar–10H2O–0.1HCl–10CO2 significantly suppressed the formation of Fe-rich oxide nodules on Fe–20Cr. Corrosion in the SO2-bearing gas resulted in (Cr, Fe, S)-rich precipitates in all alloys. The effects of gas composition and Si alloying on corrosion of both alloys are discussed in terms of transport processes within the scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid urbanization and economic development have increased the amount of solid waste sent to landfill, causing air pollution and water/soil contamination. Although recycling and composting activities divert a certain amount of waste from landfill, solid waste volume reduction is becoming increasingly critical. Waste incineration is considered a sustainable solution [1]. In this process, combustible waste is burned to produce high temperature flue gas, which is used to generate steam and electric power. Plastic and rubber products in municipal waste contain carbon, hydrogen, and other elements such as chlorine and sulphur. Incineration of these materials yields a flue gas containing mainly H2O, CO2, and lower levels of HCl and SO2.
Common chromia-forming stainless steels used in heat exchangers fail to form a protective scale in H2O [2, 3], H2O–HCl [4], and H2O–HCl–CO2 [5]. Effects of SO2 on corrosion have been reported to be beneficial in O2–H2O–SO2 at 600 °C [6] and H2O–CO2–SO2 at 650 °C [7], while detrimental effects have been observed in H2O–CO2–O2–SO2 at 550–650 °C [8, 9]. The corrosion behaviour of alloys in a complex gas mixture of H2O–HCl–CO2–SO2 is still not understood clearly, because individual gas component can have different effects, and interactions between them are possible.
Excess oxygen is used in incinerators in order to ensure fuel utilisation. However, pockets of reducing gas can form within the gas path, and such regions are particularly corrosive. This situation is explored here.
Silicon is usually present in heat-resistant alloys as a minor element (< 2 wt%). Diffusion of Si in alloys is relatively fast and its oxide SiO2 grows slowly at high temperatures. The addition of sufficient Si to chromia-forming alloys improves corrosion resistance by forming a dense SiO2 layer beneath a Cr2O3 layer in air [10], O2 [11,12,13], H2–H2O [12], air-H2O [14], CO2/CO [15], CO2 [16,17,18,19], CO2–H2O [7, 19,20,21], and SO2–O2 [22].
In this work, four model Fe–20Cr and Fe–20Cr–20Ni alloys with and without silicon were exposed to four gas mixtures Ar–10H2O, Ar–10H2O–0.1HCl, Ar–10H2O–0.1HCl–10CO2, Ar–10H2O–0.1HCl–10CO2–0.5SO2 (vol.%) at 650 °C. The aim was to investigate effects of individual gas components and Si additions on corrosion in the HCl-containing gases.
Experimental Procedures
Four model Fe–20Cr and Fe–20Cr–20Ni alloys with and without 1% Si (wt%) were prepared by arc melting pure metals Fe (99.98%, Sigma Aldrich), Cr (99.995%, Sigma Aldrich), Ni (99.95%, Alfa Aesar), and Si (99.95%, Aldrich) under a protective Ar–5%H2 (volume %) gas atmosphere, using a non-consumable electrode. The resulting buttons were homogenised at 1000 °C for 70 h in a flowing Ar–5%H2 gas. The alloy grain sizes after this annealing were 2.6 ± 2 mm for 20Cr alloys and 2.4 ± 1.8 mm for 20Cr20Ni alloys. Rectangular alloy coupons (1 ± 0.1) × (8 ± 1) × (8 ± 1) mm in size were surface ground to a 1200-grit finish and ultrasonically cleaned in alcohol prior to reaction. Alloy compositions were checked by SEM/EDX (detection limit of 0.1 wt%) and found to be within 0.2% of the nominal concentrations for both Cr and Ni, and 0.1% for Si (wt%).
Alloy specimens were reacted at 650 °C in Ar − 10H2O − (0.1HCl) − (10CO2) − (0.5SO2) mixtures (vol.%) with a linear flow rate of 1 cm/s and a total pressure of 1 atm for up to 240 h. A horizontal alumina tube furnace was used. Gases flowed at controlled rates through a flask containing water or HCl solution at a controlled temperature, yielding the desired partial pressures of H2O and HCl [23]. The detailed experimental setup is available in a previous paper [24]. Equilibrium gas compositions at 650 °C calculated using FactSage software [25] are summarised in Table 1.
No vacuum grease was used at glassware joints connecting the hydrochloric acid reservoir, because it can cause significant Si contamination for oxide scales during high temperature corrosion [24].
Weight changes of corroded samples were measured using an analytic balance (Mettler Toledo) with an accuracy of 0.01 mg. In weight gain kinetic plots shown here, each point represents a separate sample. Corroded samples were characterized by X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi-Thermo Scientific), Raman spectroscopy (Renishaw, 532 nm diode laser), scanning electron microscopy (SEM, Hitachi S3400) with an energy dispersive X-ray spectrometer (EDX, Bruker) and transmission electron microscopy (TEM, JEOL JEM-F200). The TEM samples were prepared using a focused ion beam system (FIB, FEI Nova Nanolab 200 and FEI Helios G4 PFIB) with a maximum accelerating voltage of 30 kV. In XPS measurements, the etched area was 2.5 × 2.5 mm2 and the reference etching rate was 0.17 nm/s for a standard Ta2O5 film. The diameter of the XPS analysis area was 0.5 mm. Metallographic etchants were Murakami’s solution (2 g K3Fe(CN)6 + 2 g KOH + 20 ml H2O) for staining carbides and a solution of (12 ml H2O + 4 ml HCl + 3 ml HNO3) for revealing carbides/sulphides and alloy grain boundaries.
Results
Corrosion of Fe–20Cr–(1Si) Alloys
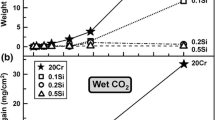
Weight gains of Fe–20Cr–(1Si) in different gases are compared in Fig. 1. As is described below, the reaction products are not uniform, and the relative weight gains are of limited significance. However, it is clear that weight uptakes of undoped Fe–20Cr were high in all gases except the SO2-bearing gas where weight gain was much smaller (Fig. 1c). It is also apparent that the addition of Si significantly reduced weight uptakes in all gases.
Fe–20Cr
Surface views of Fe–20Cr reacted in Ar–10H2O–0.1HCl for 240 h are shown in Fig. 2. Nodules formed on most of the sample surface while a thin scale was on some regions (Fig. 2a). Oxide blades were observed on both the nodules (Fig. 2b) and thin scale (Fig. 2c). The thin scale (Fig. 2c) had two different morphologies: fine perforated coarse blades at site A (Fig. 2d) and fine blades at site B (Fig. 2e). The Fe–20Cr alloy corroded in Ar–H2O and Ar–H2O–HCl–CO2 also formed mainly nodules plus regions covered by thin scales with blades (not shown).
Cross-sections in Fig. 3a–c show that the nodules on Fe–20Cr formed in the SO2-free gases Ar–H2O–(HCl)–(CO2) were porous and had similar morphology. Analyses by SEM/EDX and Raman revealed the nodules to consist of an outer Fe2O3 layer, an intermediate Fe3O4 layer, and an inner Fe3O4 + FeCr2O4 layer (Fig. 3a–e). An internal oxidation zone (IOZ) containing Cr-rich, dark-contrast precipitates (Fig. 3d, f) formed beneath the nodules. The fraction of Fe-rich oxide nodules, fi, over the whole surface was quantitatively measured based on:
where \(\sum {\ell_{{\text{i}}} }\) is the total of Fe-rich oxide nodules lengths along the line imaged in a cross-section, and \(\ell\) the total length examined. The Fe-rich nodule fractions were 66% (Ar–H2O), 79% (Ar–H2O–HCl), 84% (Ar–H2O–HCl–CO2), and 2% (Ar–H2O–HCl–CO2–SO2). It is clear that more Fe-rich nodules formed when HCl and HCl–CO2 were added to Ar–H2O, whilst the addition of SO2 to Ar–H2O–HCl–CO2 significantly suppressed nodule formation.
Cross-sections of the thin scales on Fe–20Cr formed in Ar–H2O–(HCl)–(CO2) are shown in Fig. 4a–c. The scales were relatively dense and identified by TEM/EDX (Fig. 4d, e) to be Cr2O3 containing small amounts of Fe and Si.
After reaction in the SO2-bearing gas, Fe–20Cr was covered mainly by a thin scale, together with nodules. The thin scale with blades on top was analysed by TEM/EDX to be Cr2O3 (Fig. 5a–c). Precipitates rich in (Cr, Fe, S) formed randomly in the underlying alloy. Analyses by TEM/EDX (Fig. 5a–c) and a SAD pattern (Fig. 5d) indicated the (Cr, Fe, S)-rich particle at point 2 to be CrS. Small amounts of S (0.1 at.%) and Si (0.6 at.%) were detected in the Cr2O3 scale. Fine oxide precipitates (Cr, Fe)Oy rich in Cr and Fe were observed in the alloy near the scale-alloy interface (Fig. 5a, b).
A nodule grown on Fe–20Cr (Fig. 6) analysed by TEM/EDX and SAD was found to consist of an outer Fe2O3 layer and an inner Cr2O3 layer. Analysis by TEM and EDX mapping (Fig. 6a, b) shows that (Fe, S, O)-rich grains formed randomly on top and within the nodule. Quantitative analysis (points 1, 2 in Fig. 6a, c) indicated two coarse (Fe, S, O)-rich particles to be FeSO4. Precipitates rich in (Cr, Fe, S) were observed in the underlying alloy (Fig. 6a, c).
Fe–20Cr–1Si
The Fe–20Cr–1Si alloy after oxidation in Ar–10H2O for 240 h formed a duplex scale consisting of an outer Cr2O3 layer and a thin inner SiO2 layer (Fig. 7a–c). The scale thickness was variable on the sample surface. The Cr2O3 scale layer on top of a continuous SiO2 layer was apparently thinner than that on a discontinuous SiO2 layer (Fig. 7a, b). Results of EDX analysis in Fig. 7a–c show a small amount of Si (0.5 ~ 1.1 at.%) at points 1–4 and a Si-rich cluster (11.2 at.%) at point 5 in the Cr2O3 layer. Precipitates (Cr, Fe)2O3 formed randomly in the Cr2O3 layer (points 2, 4 in Fig. 7a–c), but more iron was present in the scale where the SiO2 layer was discontinuous.
Scales on Fe–20Cr–1Si corroded in Ar–10H2O–0.1HCl (Fig. 8a) and Ar–10H2O–0.1HCl–10CO2 (Fig. 8b, c) were made up of an outer Cr2O3 layer and an inner SiO2 layer. The Cr2O3 layer contained a small amount of Si (Fig. 8d, e) and FeCr2O4 precipitates (Fig. 8b, f).
In the SO2-bearing gas, Fe–20Cr–1Si formed a thin, rather uniform scale. Analysis by TEM/EDX (Fig. 9a–d) and SAD (Fig. 9e) indicated that the scale consisted of an outer Cr2O3 layer and an inner SiO2 layer. Intragranular and intergranular (Cr, Fe, S) precipitates were observed in the underlying alloy (Fig. 9a, b). Analyses by TEM/EDX (Fig. 9c, g) and a SAD pattern (Fig. 9f) indicated a (Cr, Fe, S) precipitate at point 5 to be CrS. Intergranular (Cr, Si)xO precipitates were observed immediately beneath the scale (Fig. 9a–c). The concentration of Cr in the subsurface alloy region was reduced significantly, whereas that of Si was only slightly smaller than the original value of 2.2 at.% Si (points 7–9, Fig. 9a, b, g). Silicon was rich at the surface of the Cr2O3 layer (6.6 ~ 7.1 at%, points 1, 2, Fig. 9a, g) but only 0.4 ~ 0.8 at.% in the inner Cr2O3 region (points 3, 4). The Cr2O3 layer contained low levels of S, 0.2 ~ 1.2 at% (Fig. 9a, g).
Fe–20Cr–1Si reacted for 240 h in Ar–H2O–HCl–CO2–SO2: a BF–STEM and b DF–STEM (Dark Field STEM) cross-sections, c EDX mapping of (b), d BF-TEM high magnification image of the Cr2O3-alloy interface in (a), SAD patterns of e the whole Cr2O3 scale and f (Cr, Fe, S) (point 5) in (a), and g EDX concentrations of points 1–9 in (a)
Chemical Forms of Sulphur
Corroded surfaces of Fe–20Cr (Fe-rich nodule + Cr2O3 scale) and Fe–20Cr–1Si (Cr2O3 scale) after reaction in Ar–H2O–HCl–CO2–SO2 for 120 h were analysed by XPS to identify chemical states of S and Si (Fig. 10). Two sulfates were detected: FeSO4 in the Fe-rich nodule on Fe–20Cr and Cr2(SO4)3 in the Cr2O3 scales of both alloys. The \({\text{Fe}}_{{{\text{2p}}_{{3/2}} }}\) peak centred at 711.2 eV matches with the \({\text{Fe}}_{{{\text{2p}}_{{3/2}} }}\) binding energy in FeSO4 [26]. The \({\text{Cr}}_{{{\text{2p}}_{{3/2}} }}\) peak centred at 579 eV matches with the \({\text{Cr}}_{{{\text{2p}}_{{3/2}} }}\) binding energy in Cr2(SO4)3 [27]. The \({\text{S}}_{{{\text{2p}}_{{3/2}} }}\) peak centred at 169 eV matches with the \({\text{S}}_{{{\text{2p}}_{{3/2}} }}\) binding energy in FeSO4 [26] and Cr2(SO4)3 [27]. The Si2p peak centred at about 102.6 eV matches with the Si2p binding energy in SiO2 [28].
Fe–20Cr and Fe–20Cr–1Si reacted in Ar–H2O–HCl–CO2–SO2 for 120 h. XPS spectra of Fe2p, Cr2p, Si2p, and S2p from sulfates and SiO2 taken from the surfaces of: a, b, c Fe-rich nodule on Fe–20Cr, d, e, f thin Cr2O3 scale on Fe–20Cr, and g, h, i thin Cr2O3 scale on Fe–20Cr–1Si. j, k S2p spectra of the surface of Fe–20Cr–1Si after etching for 191 s by XPS
Another XPS analysis was performed to identify the chemical state of S within the Cr2O3 scale. In this case, the Cr2O3 scale surface of Fe–20Cr–1Si was etched for 191 s by XPS before analysis. A new \({\text{S}}_{{{\text{2p}}_{{3/2}} }}\) peak at 162.9 eV (Fig. 10k) corresponding to CrS [29] was detected together with that at 169.6 eV (Fig. 10j) from Cr2(SO4)3 [27]. No chlorine was detected by XPS on these samples.
Carbide Precipitates
The Fe–20Cr and Fe–20Cr–1Si alloys reacted in Ar–10H2O were etched with Murakami’s solution (Figs. 11a, 12a) to reveal intergranular carbide precipitates throughout. This result was unexpected because the reaction gas contained only Ar and water vapour. The carbide was identified by TEM/EDX (Fig. 11b, c) and SAD (Fig. 11d) to be Cr23C6.
In the CO2-bearing gases, both alloys also formed mainly intergranular carbide precipitates beneath the Cr2O3 scale (Figs. 11e, f and 12b, c). In addition, intragranular carbide precipitates developed beneath surface regions covered by Fe-rich scales (Fig. 11e). Depths of the dense, intragranular carbide precipitate zone beneath the Fe-rich scales of Fe–20Cr after 240 h reaction were 64 µm in Ar–H2O–HCl–CO2 gas but only 4 µm in Ar–H2O–HCl–CO2–SO2 gas.
Corrosion of Fe–20Cr–20Ni–(1Si) Alloys
Weight uptakes of Fe–20Cr–20Ni–(1Si) alloys after reactions in all gases (Fig. 13) were smaller than for the ferritic alloys. The addition of Si to Fe–20Cr–20Ni reduced the weight gains to some extent. Effects of gas compositions were not significant as the weight gains were generally very small. Partial spallation of the thin chromia scale covering much of the alloy surface was observed (Figs. 14a, 16a). As described below, the reaction products were non-uniform, consisting of a thin chromia scale together with multiple, very small nodules. Because the extent of nodule formation was difficult to measure, an average approach to quantification was attempted.
Assuming that a Cr2O3 scale covered the whole sample surface, weight uptake by the Cr2O3 scale formation is calculated from
where \(\rho_{{{\text{Cr}}_{{2}} {\text{O}}_{{3}} }}\) and \(M_{{{\text{Cr}}_{{2}} {\text{O}}_{{3}} }}\) are density and molar mass of Cr2O3, \(\delta_{{{\text{Cr}}_{{2}} {\text{O}}_{{3}} }}\) the average measured chromia scale thickness, \(M_{{\text{O}}}\) molar mass of oxygen, and A sample surface area. Since the Cr2O3 scale was thin and the amount of (C, S)-containing products was small, weight uptake due to Fe-rich nodule formation was approximated by
where \(\frac{{m_{{\text{O}}}^{{{\text{nodule}}}} }}{A}\) and \(\frac{{\Delta m_{{}}^{{{\text{sample}}}} }}{A}\) are weight uptakes of Fe-rich nodules and total measured weight gains of the sample. The calculated \(\frac{{m_{{\text{O}}}^{{{\text{nodule}}}} }}{A}\) values (mg/cm2) for the two alloys (Fe–20Cr–20Ni, Fe–20Cr–20Ni–1Si) after 240 h reaction were (0.01, 0.02) in Ar–H2O, (0.07, 0.06) in Ar–H2O–HCl, (0.05, 0.05) in Ar–H2O–HCl–CO2, and (0.12, 0.09) in Ar–H2O–HCl–CO2–SO2. It is clear that the formation of Fe-rich nodules was promoted on both austenitic alloys when adding HCl, CO2, and SO2 to Ar–H2O.
Fe–20Cr–20Ni
The undoped Fe–20Cr–20Ni alloy formed a thin scale (sites A and B in Fig. 14a) plus small nodules in Ar–10H2O–0.1HCl. Scale spallation occurred locally on the sample surface. Oxide blades were observed on the nodules (Fig. 14b). Perforated, coarse blades formed randomly together with fine blades on the thin scale at site A (Fig. 14c) while only fine blades formed at other regions of the thin scale (site B, Fig. 14d). In Ar–10H2O and Ar–10H2O–0.1HCl–10CO2, the Fe–20Cr–20Ni alloy also formed thin scales and nodules (not shown).
The thin scales on Fe–20Cr–20Ni reacted in H2O–(HCl)–(CO2) were analysed by TEM/SEM (Fig. 15a-c) and EDX (not shown) to be Cr2O3. Small amounts of (Si, Fe, Ni) were detected in the Cr2O3 scales: (Si 0.4, Fe 2.5, Ni 0.7, at.%) in H2O and (Si 2.1, Fe 1.9, Ni 0.2, at.%) in H2O–HCl. A cross-section of one nodule formed in H2O–HCl is shown in Fig. 15d. Analyses by TEM/EDX (Fig. 15e, f) and a SAD pattern (not shown) revealed the nodule to be made up of an outer (Fe, Cr) rich oxide layer, an intermediate Fe2O3 layer, an inner (Fe, Ni, Cr)Ox layer, and an IOZ. Large protrusions of Cr2O3 were observed in the IOZ (points 5–6, Fig. 15d, f).
Surface views of Fe–20Cr–20Ni corroded in Ar–H2O–HCl–CO2–SO2 in Fig. 16a show two different morphologies: one region covered by granular Fe-rich oxides (Fig. 16b, c) and the other by Cr-rich oxides (Fig. 16b, d). Oxide blades had formed on top of the Cr-rich region (Fig. 16f). Scale spallation occurred locally. Silicon impurity was detected on the sample surface by EDX analysis (Fig. 16c, d).
Cross-sections of the alloy after reaction in S-bearing gas revealed morphologies which differed from those produced in S-free gases. A TEM cross-section of the Fe-rich region on Fe–20Cr–20Ni corroded in H2O–HCl–CO2–SO2 is shown in Fig. 17a. Analyses by EDX (Fig. 17b, c) and SAD (Fig. 17d) revealed the scale to consist of an outer Fe2O3 layer and an inner Cr2O3 layer. A deformation zone beneath the scale was depleted in Cr and enriched in Ni (Fig. 17a, b). Intragranular and intergranular (Cr, Fe, S)-rich precipitates were observed in the deformation zone (Fig. 17a, b). Results by SAD (Fig. 17f) and EDX analysis (Fig. 17c) indicated the (Cr, Fe, S)-rich precipitate at point 6 to be CrS. Small amounts of Si ((0.1 – 0.7 at%,) and S (0.3 – 0.4 at.%) were also detected in the scale (points 1–4, Fig. 17a, c).
Fe–20Cr–20Ni reacted for 240 h in Ar–H2O–HCl–CO2–SO2: a BF-STEM cross-section of the Fe-rich region in Fig. 16b, b EDX mapping of (a), c EDX concentrations of points 1–6 in (a), SAD patterns of (d) the Fe2O3 outer layer and f a (Cr, Fe, S)-rich precipitate (point 6) in (a)
The thin Cr-rich scale on Fe–20Cr–20Ni corroded in H2O–HCl–CO2–SO2 was identified by TEM/EDX (Fig. 18a–c) and SAD (Fig. 18d) as Cr2O3. The outer Cr2O3 scale layer on this Si-free alloy was quite rich in Si (3.7 – 5.3 at.%, points 1–2) and the inner layer contained 0.3 – 0.4 at.% Si (Fig. 18a, c). A small amount of S (0.1 – 0.5 at.%) was also detected in the Cr2O3 scale (Fig. 18a, c).
Fe–20Cr–20Ni reacted for 240 h in Ar–H2O–HCl–CO2–SO2: a BF-TEM cross-section of the Cr-rich region in Fig. 16b, b EDX mapping, c EDX concentrations of points 1–4 in (a), and d SAD pattern of the whole Cr2O3 scale in (a)
Fe–20Cr–20Ni–1Si
The Fe–20Cr–20Ni–1Si alloy formed a thin Cr-rich scale and Fe-rich nodules in all gases, just as the undoped Fe–20Cr–20Ni alloy did.
Analysis of the Cr-rich scales formed on Fe–20Cr–20Ni–1Si in Ar–H2O–(HCl)–(CO2) by TEM/EDX (Fig. 19a–e) and SAD (Fig. 19f) showed them to consist of an outer Cr2O3 layer and an inner, amorphous SiO2 layer. The Cr2O3 layer formed in H2O–HCl–CO2 contained a small amount of Si (1.5 – 1.9 at.%, points 1–2, Fig. 19c, d).
In the SO2-bearing gas, results of TEM/EDX (Fig. 20) show that the Fe-rich scale on Fe–20Cr–20Ni–1Si was made up of an outer Fe2O3 layer and an inner Cr2O3 layer. Silicon was enriched locally beneath the scale (Fig. 20b). An intergranular (Cr, Fe, S)-rich precipitate was observed in a Cr-depleted deformation zone underneath the scale. Substantial porosity developed at the scale-alloy interface. Small amounts of Si (0.1 – 0.4 at.%) and S (0.2 at.%) were detected in the scale (Fig. 20c).
A BF-TEM (Bright-Field TEM) cross-section of the Cr-rich scale region on Fe–20Cr–20Ni–1Si is shown in Fig. 21a. Analysis by EDX (Fig. 21b, c) and SAD (Fig. 21d, e) revealed this scale to consist of an outer Cr2O3 layer and a discontinuous inner, amorphous SiO2 layer. Random Fe2O3 grains had formed on top of the Cr2O3 layer (Fig. 21b, c). Coarse pores were observed in the Cr2O3 layer (Fig. 21a). Results of EDX analysis show small amounts of Si (0.1 – 0.8 at.%) and S (0.1 – 0.2 at.%) in the Cr2O3 layer (Fig. 21a, c).
Carbide Precipitates
The undoped Fe–20Cr–20Ni and Fe–20Cr–20Ni–1Si oxidised in Ar–10H2O were etched with HCl–HNO3 solution (Fig. 22a–c) and Murakami’s solution (Fig. 23a), revealing intergranular Cr-rich carbide precipitates. In CO2-bearing gases, both alloys also formed intergranular Cr-rich carbide precipitates (Figs. 22d–f and 23b–d). Corrosion in SO2-bearing gas resulted in intergranular (Cr, Fe, S)-rich precipitates in Fe–20Cr–20Ni (Fig. 22g, h) and (Cr, Si, Fe, S)-rich precipitates in the deformation zone of Fe-20Cr-20Ni-1Si (Fig. 23e–f).
Cross-sections of Fe–20Cr–20Ni (etched) after reactions for 240 h: a SE–SEM image (H2O), b high magnification image of (a), c EDX line profiles along A–B in (b), d SE-SEM image (H2O–HCl–CO2), e SE-SEM image (H2O–HCl–CO2–SO2), f high magnification image of (e), g SE-SEM image of a S-rich particle formed in H2O–HCl–CO2–SO2, and (h) EDX line profiles along C–D in (g)
Cross-sections of Fe–20Cr–20Ni–1Si (etched) after reactions for 240 h: a SE–SEM image (H2O), b SE–SEM image showing carbide precipitates (H2O–HCl–CO2–SO2), c high magnification image of (b), d EDX line profiles along A–B in (c), e SE-SEM image showing a (Cr, S)-rich precipitate (H2O–HCl–CO2–SO2), and f EDX line profiles along C–D in (e)
Chromia Layer Thicknesses
Average Cr2O3 layer thicknesses grown on alloys reacted for 240 h in four gases (Fig. 24) were measured in TEM and SEM images. For each alloy, the Cr2O3 scale layers grown in Ar–10H2O were thinnest, and the thickest layer grew in the SO2-bearing gas. Additions of Si to both ferritic and austenitic alloys significantly reduced the growth rates of the Cr2O3 layers in H2O–(HCl)–(CO2) but not in H2O–HCl–CO2–SO2, where instead an acceleration in chromia scaling resulted.
Discussion
The ferritic Fe–20Cr alloy failed to form a continuous, protective Cr2O3 scale in all gases, allowing growth of Fe-rich oxide nodules. However, nodule growth was to a large extent suppressed in the SO2-bearing gas. The austenitic Fe–20Cr–20Ni alloy reacted much more slowly than the ferritics in S-free gases, forming Cr2O3 scales and thin Fe-rich oxide nodules. The addition of Si to Fe–20Cr–(20Ni) markedly reduced Cr2O3 scale thickening rates in all gases, except the SO2-bearing gas (Fig. 24). The main corrosion products are summarised in Table 2.
Corrosion in H2O–HCl–CO2–SO2 was complex, and effects of individual gas components are now considered.
Effects of HCl and HCl–CO2
The undoped Fe–20Cr alloy underwent breakaway corrosion in Ar-10H2O, forming Fe-rich nodules (Fig. 3a) and Cr2O3 scales (Fig. 4a). Additions of either HCl or HCl–CO2 to Ar–10H2O did not change this basic morphology, but seemed to increase the total thickness of inner oxide plus IOZ in non-protective regions (Fig. 3a–c), and Cr2O3 scale thickening for the protective areas (Table 2, Fig. 24).
The presence of HCl in the gas has obvious effects on the scale microstructure. The Fe3O4 layers formed in HCl-bearing gases were more porous than that in Ar–H2O (Fig. 3a–c). The layer thickness ratio X(Fe3O4)/X(Fe2O3) in HCl-bearing gases was greater than that in Ar–H2O (Fig. 3a–c). The inner Fe3O4 + FeCr2O4 layer was porous.
The addition of HCl in the gas could lead to the volatilization of iron oxide as iron chloride and its reoxidation due to the “active oxidation” mechanism [30, 31], producing porous Fe-rich oxides. Detailed analysis of this process has been carried out in [5] for Fe–Cr alloys in H2O–HCl–CO2 gas at 650 °C. The key finding was that FeCl2(g) was predicted to form within the iron oxide scale layer at a sufficient partial pressure to account for volatilisation. The observed more porous structure in the external iron oxide scales in HCl-containing gases (Fig. 3b, c) could be attributed to this effect which facilitates gas access and an increase in interior oxygen activity. As a result, oxygen permeability increases and therefore total inner oxide plus IOZ thicknesses increase (Fig. 3a–c).
Faster Cr2O3 scale growth could be due to either a higher oxygen partial pressure (\(p_{{{\text{O}}_{{2}} }}\)) and/or faster Cr transport through the scale. The former possibility is not supported because the \(p_{{{\text{O}}_{{2}} }}\) values of Ar–10H2O and Ar–10H2O–0.1HCl–(10CO2) were essentially the same (Table 1). The latter is now considered.
Exposure of Fe–20Cr to HCl-bearing wet gases can result in oxidation and chloridation.
The calculated Gibbs free energy of reaction (8) \({\Delta G}^{{\text{o}}} = 518.5\;{\text{(kJ)}}\) [25], indicating that this reaction is not thermodynamically favoured on the Cr2O3 scale surface where \(p_{{{\text{O}}_{{2}} }}\) is relatively high.
If Cl2 can penetrate to the Cr2O3 scale-alloy interface with its low \(p_{{{\text{O}}_{{2}} }}\), formation of volatile chloride species is possible [25] via the reaction
The maximum possible \(p_{{{\text{CrCl}}_{{2}} }}\) calculated using an estimated activity of Cr in Fe-20Cr (aCr ≈ 0.21) and partial pressure of Cl2 (\(p_{{{\text{Cl}}_{{2}} }}\) = 4.1 × 10–10 atm) in Ar-10H2O-0.1HCl at 650 °C is more than 1 atm, showing that formation of volatile CrCl2 can occur.
When volatile CrCl2 species were transported to the scale surface, oxidation of CrCl2 to form Cr2O3(s) by the reverse of Eq. (8), accelerated scale thickening, following the “active oxidation” mechanism [30].
Additional support for chromium volatilisation is provided by the observation of perforated coarse blades on Fe–20Cr (Fig. 2d) and Fe–20Cr–20Ni (Fig. 14c) only in HCl-bearing gases. These porous, coarse oxide blades with different growth orientations had poor contact with the underlying scale, but still grew faster than the surrounding fine, dense blades (Fig. 2d, e). On this basis, it is suggested that the coarse blades and the underlying Cr2O3 scale received an additional supply of volatile Cr species, together with slow, solid-state Cr transport from the alloy to the scale surface. Similar perforated coarse blades have been reported for Fe–30Cr [24] and pure Cr [32], all in HCl-containing gases.
The addition of CO2 to Ar–10H2O–0.1HCl slightly increased the Cr2O3 scale growth rate for Fe–20Cr but had no clear effect for Fe–20Cr–20Ni (Fig. 24).
Effects of SO2
Failure of Cr2O3 Scales
Corrosion of undoped Fe–20Cr and Fe–20Cr–20Ni formed a mixture of Fe-rich nodules and thin Cr2O3 scales in all gases, the austenitic alloy being markedly more resistant to corrosion (Table 2). The negative effects of water vapour [2, 3], HCl [4] and CO2 [17, 19] on the ability of chromia-formers to resist breakaway corrosion are well known. All are due to inward diffusion of secondary reactants into and through the scale, and consequent modification of its mass transport properties. As is clear from the present results, combinations of these reactants are even more destructive, an effect due to promotion of Fe-rich nodule formation.
What is of especial interest here is the finding that the addition of SO2 to Ar–10H2O–0.1HCl–10CO2 reduced the weight uptakes of Fe–20Cr markedly (Fig. 1c), but had no significant effect on Fe–20Cr–20Ni (Fig. 13c). Failure of the chromia scales may be caused by chemical and/or mechanical factors. The former is now considered.
The Cr2O3 scales grew faster on all alloys in the SO2-bearing gas than those in other gases (Fig. 24), resulting in more Cr-depletion in the underlying alloy. However, the Cr2O3 scale continued to grow in the SO2-bearing gas up to 240 h. This indicates that the alloy Cr concentration was sufficient to support Cr2O3 scale growth, at least locally, in all gases in this time domain.
The oxygen partial pressures of Ar–H2O–(HCl)–(CO2) gases were small (Table 1) but thermodynamically sufficient to oxidize both Cr and Fe. Oxidation of Fe–20Cr at the initial stage of reaction would form nuclei of both Cr2O3 and Fe-rich oxides. The final formation of either protective chromia scales or non-protective Fe-rich oxide nodules depends on the competitive effect of the oxide growth, which is affected by many factors and can be quite different in different surface areas. The addition of SO2 to Ar–H2O–HCl–CO2 increased the Cr2O3 scale growth on Fe–20Cr (Fig. 24) and reduced the weight uptake (Fig. 1c) by suppressing the formation of Fe-rich oxide nodules. The same results have been reported for Fe–20Cr corroded in Ar–20CO2–20H2O with/without 0.5SO2 (vol.%) at 650 °C, but with no explanation [7, 33].
The changed outcome could in principle reflect a different result of competition between initial chromia and Fe oxide formation, but the altered nature of the iron oxide regions formed in SO2-bearing gas (hematite on top of a chromia layer rather than a multilayered Fe-rich scale) suggests otherwise. It is suggested here that chromia forms over the entire alloy surface in the early stages of reaction, but is subsequently subject to limited local failure. Another possibility is that the alloy fails to form a uniform Cr2O3 scale over the whole sample surface in the low \(p_{{{\text{O}}_{{2}} }}\) gases in the initial stage, leading to development of local Fe-rich nodules.
It is recognised that all mass transport processes in a chromia scale are concentrated at its grain boundaries at this low temperature. Indeed, as has recently been shown [34], sulphur passes through a growing chromia scale along the oxide grain boundaries. In this position, the sulphur can affect the rate at which metal and other species diffuse via the grain boundary surfaces. As has already been shown [35, 36], carbon also penetrates chromia scales via their grain boundaries. However, when SO2 is present, as seen here (Fig. 11e, f), carbon penetration is reduced, and the alloy forms far fewer carbide precipitates. This is beneficial, as more solute chromium remains in the ferrite, which can stabilize the chromia scale.
An additional effect can arise via interaction between S and Fe on the oxide grain boundaries. Iron has been shown [36] to concentrate in Cr2O3 scale within its grain boundaries, forming narrow regions of composition approximating to the spinel FeCr2O4. The spinel phase has a relatively high diffusivity for Fe, and these regions provide a possible pathway for outward iron transport, subsequent Fe-rich oxide formation and loss of protection. Sulphur on the grain boundaries could immobilize iron, preventing spinel formation and reducing greatly the ability of the scale to transmit iron. In such a situation where outward Fe diffusion is for this reason much slower, its oxidation at the surface to form the slow-diffusing hematite phase accounts for the changed scale morphology observed in the presence of SO2-bearing gas.
Oxide growth stress could also affect chromia scale performance. A main factor that can reduce scale stress is the scale plasticity. The Cr2O3 scale formed in the SO2-bearing gas contained additional sulfur-species (Figs. 5a, c, 9a, g) which is expected to affect their mechanical behaviour and thereby the scale's plasticity. This might improve the mechanical stability of the scale, and its resistance to cracking, gas access and breakaway. Present results do not provide information on this possibility.
Sulfidation
Exposure of Fe–20Cr and Fe–20Cr–20Ni to the SO2-bearing gas resulted in both oxidation and sulfidation. On the sample surface, XPS analysis detected FeSO4 on Fe-rich nodules (Figs. 6, 10a, b) and Cr2(SO4)3 on Cr2O3 scales (Fig. 10d, e). Similar results have been reported for sulphates on 304L steel corroded in O2–40H2O–0.01SO2 at 600 °C [6] and Fe–20Cr corroded in Ar–20CO2–20H2O–0.5SO2 at 650 °C [33]. Predominance phase diagrams calculated for the Fe–S–O and Cr–S–O systems at 650 °C [25] show that only Fe2O3 and Cr2O3 are stable in contact with equilibrium oxygen and sulphur pressures of Ar–10H2O–0.1HCl–10CO2–0.1SO2. However, the experimental results are not consistent with this prediction. It is clear that the sulphates were not at equilibrium with the reaction gas.
This can be understood in rather general terms, on the basis that the important reactant species is SO2, not the much less abundant O2 or S2. This factor, plus the ability of sulphur to adsorb preferentially on scale surfaces together with the rather high stability of solid metal sulfates can enable formation of sulphate phases which are metastable with respect to the bulk gas composition. More detailed discussion of formation of sulphates on the scale surface and (Cr, Fe)-rich sulfides in the underlying alloy by SO2-bearing gas is available elsewhere [37].
Effects of Si on Oxidation and Sulfidation
The Si addition to Fe–20Cr significantly improved corrosion resistance in Ar–H2O–(HCl)–(CO2)–(SO2) by suppressing the formation of Fe-rich oxide nodules (Fig. 1a–c). The benefit of Si was provided by the formation of a thin, slow growing amorphous SiO2 layer at the scale-alloy interface (Figs. 7, 8), acting as a diffusion barrier for the outward diffusion of Cr and Fe from the underlying alloy to the scale. For this reason, the outer Cr2O3 scale layer grew slower on Fe–20Cr–1Si in the SO2-free gases (Fig. 24).
It was suggested earlier in this paper that growth stresses may be one of the factors for the Cr2O3 scale failure on undoped Fe–20Cr. The slower Cr2O3 scale layer growth on Fe–20Cr–1Si increased the incubation time for the Cr2O3 layer cracking. When cracks formed locally through the outer Cr2O3 layer, the inner SiO2 layer beneath the cracks provided an additional protection to the alloy until the cracks were healed. Therefore, the duplex scale of Cr2O3 and SiO2 layers lasted longer and was more protective than the Cr2O3 scale.
The addition of Si to Fe–20Cr–20Ni had a minor benefit on weight uptakes in all gases (Fig. 13). The Cr2O3 scales grew slower on Fe–20Cr–(20Ni)–1Si than those on the undoped alloys in all gases, except the SO2-bearing gas (Fig. 24). It has been reported that the addition of Si to Fe–20Cr–(20Ni) significantly reduced Cr2O3 scale growth rates in Ar–20CO2–20H2O at 650 °C [19] but had no effect in Ar–20CO2–20H2O–0.1SO2 [7]. In the SO2–free gas, the amorphous SiO2 layer at the scale-alloy interface (Figs. 7, 8, 19) acted to reduce the outward diffusion of Cr, leading to thinner, outer Cr2O3 layers. However, this benefit of Si depends on continuity and thickness of the SiO2 layer. The Cr2O3 layer grown on top of a continuous, thick SiO2 layer was thinner than that on a discontinuous, thin SiO2 layer (Fig. 7a, b).
In the SO2-bearing gas, the formation of S-rich precipitates at the scale-alloy interface interrupted coalescence and growth of the SiO2 layer (Fig. 9a–c). A layer containing a mixture of S-rich phases and SiO2 grains, with more grain boundaries/phase interfaces allowing fast diffusion, is less protective than a continuous, amorphous SiO2 layer. For this reason, the addition of Si had no effect on the Cr2O 3 scale growth rate in the SO2-bearing gas.
The addition of Si had no effect on the outward Cr transport from the underlying alloys in the SO2-bearing gas, showing that the SiO2 layer was not an effective barrier at the scale-alloy interface in the SO2 corrosion. On the same basis, it can be concluded that the inward sulphur transport to the alloys was not impeded, resulting in (Cr, Fe, S)-rich precipitates in Fe–20Cr–(20Ni)–1Si (Figs. 9, 20 and 23e, f).
Silicon Contamination During Corrosion
The Si-free Fe–20Cr–(20Ni) alloys formed scales/nodules containing Si impurity in all gases (Figs. 4d, e, 5c, 15f, 16c, d, 17 and 18). Analysis by XPS revealed the Si impurity to be SiO2 on both Fe-rich oxide nodules (Fig. 10c) and Cr2O3 scales (Fig. 10f). The Si contamination is deduced to be caused by Si impurity in deionised liquid water and the HCl solution used to form water vapour and HCl gas [24, 38], resulting in volatile Si species in the reaction gases. This Si contamination seems to be unavoidable during corrosion in wet gases because tap water is not absolutely pure and it is nearly impossible for water deionisers to filter impurities completely [38] As a result, scales and nodules on all alloys in this study were contaminated by Si in all gases.
Carburisation
All alloys precipitated carbides underneath their scales in all Ar–H2O–(HCl)–(CO2)–(SO2) gases (Figs. 11, 12, 22 and 23). The formation of Cr23C6 carbide precipitates in the model alloys during oxidation in Ar–H2O was not expected (Figs. 11a–d, 12a, 22a and 23a) because this gas contained no CO2. Therefore, it is concluded that the model alloys contained carbon impurity, forming internal carbide precipitates at high temperature.
The carbon impurity was suggested to be from pure metals used to produce model alloys. The pure metals in this study had a high purity: Fe (99.98%, Sigma Aldrich), Cr (99.995%, Sigma Aldrich) and Ni (99.95%, Alfa Aesar). According to the manufacturers, the compositional limits were quoted only a trace metal basis using ICP analysis, because no other analytical methods to analyse non-metallic elements like carbon had been used.
The Fe-20Cr alloy formed only a small amount of intergranular carbide precipitates beneath an Fe-rich scale in Ar–H2O (Fig. 11a) but a marked amount of intergranular and intragranular carbide precipitates in Ar–H2O–HCl–CO2 (Fig. 11e). Clearly, the significant carburisation beneath the non-protective Fe-rich scale was caused by the additional carbon supply from CO2, and the decreased ability of Fe-rich oxides to prevent carbon ingress. The Fe-20Cr alloy formed mainly a protective Cr2O3 scale in the SO2-bearing gas (Fig. 11f). Fewer carbide precipitates were formed in this gas because chromia provides a superior barrier to carbon transport [39].
The austenitic Fe–20Cr–20Ni alloy formed only intergranular carbide precipitates in all gases (Figs. 22, 23). Any effects of individual gas components (H2O, HCl, CO2, SO2) on carburisation of Fe–20Cr–20Ni are unknown because there was no significant difference in terms of the amount and morphology of the carbide precipitates.
The addition of Si to Fe–20Cr suppressed the formation of Fe-rich nodules and therefore reduced the amount of carbide precipitates in the underlying alloy (Fig. 11e, 12b). In the case of austenitic Fe–20Cr–20Ni, the addition of Si had no clear effect on carburisation which was in any event already negligible (Figs. 22, 23).
The addition of SO2 to Ar–H2O–HCl–CO2 suppressed formation of Fe-rich oxide nodules on Fe–20Cr, and therefore carburisation was significantly reduced (Fig. 11e,f).
Conclusions
Model Fe–20Cr alloy underwent breakaway corrosion in all gases, forming a thin Cr2O3 scale and Fe-rich oxide nodules. The addition of HCl or HCl–CO2 to Ar–H2O led to the enhanced chromia thickening and more extensive formation of Fe-rich nodules due to the well-known active oxidation mechanism.
The addition of SO2 to Ar–H2O–HCl–CO2 affected Cr2O3 grain boundary transport, resulting in faster Cr2O3 scale growth, slower outward iron diffusion, almost complete suppression of Fe-rich nodule formation and greatly improved Fe–20Cr corrosion resistance.
The austenitic Fe–20Cr–20Ni had better corrosion resistance than Fe–20Cr although it also formed a partly protective thin Cr2O3 scale and non-protective Fe-rich oxide nodules. The Fe-rich nodules grew much slower on Fe–20Cr–20Ni than those on Fe–20Cr.
The addition of Si to Fe–20Cr significantly improved corrosion resistance of Fe–20Cr by forming an additional amorphous SiO2 layer at the Cr2O3 scale layer-alloy interface in all gases, suppressing the formation of Fe-rich nodules.
The addition of Si to Fe–20Cr–(20Ni) reduced the Cr2O3 scale growth because of the continuous silica layer formation in all gases, except the SO2-bearing gas where only particulate silica was formed and no benefit was achieved.
Corrosion in the SO2-bearing gas resulted in (Cr, Fe, S)-rich precipitates in all alloys beneath their scales.
The Cr2O3 scales were contaminated with SiO2 because of volatile Si impurities from water vapour/HCl in the reaction gases.
All alloys developed intergranular carbides in all gases, because carbon impurity in the model alloys was sufficient to form Cr-rich carbide precipitates at high temperature. The undoped Fe–20Cr alloy formed more carbide precipitates beneath Fe-rich nodules than underneath a more protective Cr2O3 scale in CO2-containing gas because of additional carbon supply from the gas. The addition of SO2 reduced the amount of carbide precipitates in Fe–20Cr because the formation of Fe-rich nodules was suppressed markedly. The effect of alloy Si in Fe–20Cr was to prevent significant carburisation by suppressing Fe-rich nodule formation, and by providing an additional barrier to inward carbon transport in the form of an amorphous SiO2 scale layer.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
United States Environmental Protection Agency Report: “National Overview: Facts and Figures on Materials, Wastes and Recycling”, https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials.
C. T. Fujii and R. A. Meussner, Journal of The Electrochemical Society 110, 1195 (1963 ).
E. Essuman, G. H. Meier, J. Zurek, M. Hansel, and W. J. Quadakkers, Oxidation of Metals 69, 143 (2008).
X. Zheng and R. A. Rapp, Oxidation of Metals 48, 553 (1996).
K. K. Aye, J. Zhang, and D. J. Young, Oxidation of Metals 94, 51 (2020).
A. Jardnas, J. E. Svensson, and L. G. Johansson, Oxidation of Metals 69, 249 (2008).
C. Yu, T. D. Nguyen, J. Zhang, and D. J. Young, Journal of The Electrochemical Society 163, C106 (2016).
P. Huczkowski, D. J. Young, T. Olszewski, A. Chyrkin, and W. J. Quadakkers, Oxidation of Metals 89, 651 (2018).
R. P. Oleksak, J. H. Tylczak, G. R. Holcomb, and O. N. Dogan, Corrosion Science 164, 108316 (2020).
B. Li and B. Gleeson, Oxidation of Metals 65, 101 (2006).
A. M. Huntz, V. Bague, G. Beauple, C. Haut, C. Severac, P. Lecour, X. Longaygue, and F. Ropital, Applied Surface Science. 207, 255 (2003).
L. Mikkelsen, S. Linderoth, and J. B. Bilde-Sørensen, Materials Science Forum 461–464, 117 (2004).
F. H. Stott, G. J. Gabriel, F. I. Wei, and G. C. Wood, Materials and Corrosion 38, 521 (1987).
S. Mahboubi, H. S. Zurob, G. A. Botton, and J. R. Kish, Corrosion Science 143, 376 (2018).
H. E. Evans, D. A. Hilton, R. A. Holm, and S. J. Webster, Oxidation of Metals 19, 1 (1983).
M. J. Bennett, J. A. Desport, and P. A. Labun, Proceedings of the Royal Society A 412, 223 (1987).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 81, 549 (2014).
T. D. Nguyen, J. Zhang, and D. J. Young, Corrosion Science 130, 161 (2018).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 87, 541 (2017).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 83, 575 (2015).
T. D. Nguyen, J. Zhang, and D. J. Young, Corrosion Science 170, 108702 (2020).
S. N. Basu and G. J. Yurek, Oxidation of Metals 35, 441 (1991).
R.H. Perry, D.W. Green, J.O. Maloney, Perry’s Chemical Engineers’ Handbook, 7th ed. McGraw-Hill, (1997)
T. D. Nguyen, J. Zhang, and D. J. Young, Corrosion Science 209, 110808 (2022).
FactSage 8.1. Database: FactPS
A. P. Grosvenor, B. A. Kobe, M. C. Biesinger, and N. S. McIntyre, Surface and Interface. Analysis 36, 1564 (2004).
M. C. Biesinger, C. Brown, J. R. Mycroft, R. D. Davidson, and N. S. McIntyre, Surface and Interface. Analysis 36, 1550 (2004).
D. A. Stephenson and N. J. Binkowski, Journal of Non-Crystalline Solids 22, 399 (1976).
P. Marcus and J. M. Grimal, Corrosion Science 31, 377 (1990).
M. J. McNallan, W. W. Liang, J. M. Oh, and C. T. Kang, Oxidation of Metals 17, 371 (1982).
C. Schwalm and M. Schutze, Materials and Corrosion 51, 34 (2000).
T. D. Nguyen, J. Zhang, and D. J. Young, High Temperature Corrosion of Materials 100, 1 (2023).
C. Yu, J. Zhang, and D. J. Young, Oxidation of Metals 90, 97 (2018).
C. Sha, L. Yang, J. M. Cairney, J. Zhang, and D. J. Young, Corrosion Science 222, 111410 (2023).
D. J. Young, T. D. Nguyen, P. Felfer, J. Zhang, and J. M. Cairney, Scripta Materialia 77, 29 (2014).
T. D. Nguyen, A. L. Fontaine, L. Yang, J. M. Cairney, J. Zhang, and D. J. Young, Corrosion Science 132, 125 (2018).
F. Gesmundo, D. J. Young, and S. K. Roy, High Temperature Materials and Processes 8, 149 (1989).
T. D. Nguyen, J. Zhang, and D. J. Young, Oxidation of Metals 97, 559 (2022).
T. Gheno, D. Monceau, J. Zhang, and D. J. Young, Corrosion Science 53, 2767 (2011).
Acknowledgements
Financial support from the Australian Research Council’s Discovery Program is gratefully acknowledged.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
TDN: Methodology, Formal analysis, Investigation, Writing—Original draft, Visualization. JZ: Supervision, Methodology, Formal analysis, Validation, Writing—Review & editing, Funding acquisition, Project administration, DJY: Supervision, Methodology, Formal analysis, Validation, Writing—Review & editing, Funding acquisition, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, T.D., Zhang, J. & Young, D.J. Effects of Si and SO2 on Corrosion of Fe–20Cr and Fe–20Cr–20Ni Alloys in Reducing Waste Combustion Gases. High Temperature Corrosion of mater. 100, 621–653 (2023). https://doi.org/10.1007/s11085-023-10200-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-023-10200-3