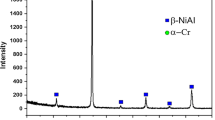
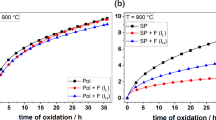
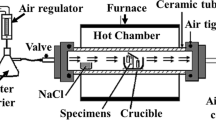
Abstract
Though Ni-based superalloys show a high oxidation and corrosion resistance, coatings can still improve these properties, especially if used at temperatures up to 1000 °C. Here, a coating was prepared by applying a boehmite-sol via dip-coating and a subsequent heat treatment at 600 °C for 30 min. To evaluate the coating, the oxidation behavior of bare and alumina-coated Ni-base alloy Inconel 625 in air at 900 °C was studied for up to 2000 h. Electron microscopy studies of sample surfaces and cross sections showed that (1) in the 3.5–6.3 µm-thick scale formed on the bare alloy, Fe and Ni are located as fine precipitates at the grain boundaries of the chromia-rich scale, (2) Ni and Ti are concentrated to a minor degree at the grain boundaries of the scale, too; and for the coated sample: (3) the only 1.8-µm-thick sol–gel alumina coating slows down the formation of chromia on the alloy surface and reduces the outward diffusion of the alloy constituents. The protective effect of the coating was evidenced by (1) diminished chromium diffusion at grain boundaries resulting in less pronounced string-like protrusions at the outer surface of the coated IN 625, (2) formation of a Cr-enriched zone above the alloy surface which was thinner than the scale on the uncoated sample, (3) lower extension in depth of Cr depletion in the superficial zone of the alloy surface of the coated sample in comparison with that region of the uncoated one, and (4) a narrower zone of formation of Kirkendall pores.
Graphical Abstract















Similar content being viewed by others
References
C. H. White, in The Development of Gas Turbine Materials, ed. G. W. Meetham (Springer, Dordrecht, 1981), p. 89.
D. Seo, M. Sayar, and K. Ogawa, Surface & Coatings Technology 206, 2851 (2012).
J. Liu, D. Dyson, and E. Asselin, Oxidation of Metals 86, 135 (2016).
D. M. Gorman, R. L. Higginson, H. Du, G. McColvin, A. T. Fry, and R. C. Thomson, Oxidation of Metals 79, 553 (2013).
D. Fantozzi, V. Matikainen, M. Uusitalo, H. Koivuluoto, and P. Vuoristo, Surface & Coatings Technology 318, 233 (2017).
T. Sugama, Journal of Sol-Gel Science and Technology 12, 35 (1998).
T. Sugama, Surface and Coatings Technology 106, 106 (1998).
H. Cho, D. M. Lee, J. H. Lee, K. H. Bang, and B. W. Lee, Surface & Coatings Technology 202, 5625 (2008).
M. Dressler, M. Nofz, R. Saliwan-Neumann, I. Dörfel, and M. Griepentrog, Thin Solid Films 517, 786 (2008).
M. Dressler, M. Nofz, I. Dörfel, and R. Saliwan-Neumann, Surface & Coatings Technology 202, 6095 (2008).
M. Nofz, I. Dörfel, R. Sojref, N. Wollschläger, M. Mosquera-Feijoo, and A. Kranzmann, Journal of Sol-Gel Science and Technology 81, 185 (2017).
M. Nofz, I. Dörfel, R. Sojref, et al., Oxidation of Metals 89, 453 (2018).
www.specialmetals.com; downloaded 14 June 2018.
L. Kumar, R. Venkataramani, M. Sundararaman, P. Mukhopadhyay, and S. P. Garg, Oxidation of Metals 45, 221 (1996).
A. Chyrkin, Oxidation of Metals 75, 143 (2011).
E. N’dah, M. P. Hierro, K. Borrero, and F. J. Pérez, Oxidation of Metals 68, 9 (2007).
J. Zurek, D. J. Young, E. Essuman, et al., Materials Science and Engineering A 477, 259 (2008).
M. D. Mathew, P. Paraweswaran, and K. Bhanu Sankara Rao, Materials Characterization 59, 508 (2008).
P. Petrzak, K. Kowalski, and M. Blicharski, Acta Physica Polonica A 130, 1041 (2016).
R. P. Oleksak, C. S. Carney, G. R. Holcomb, and Ö. N. Doğan, Oxidation of Metals 90, 27 (2018).
M. Abbasi, D.-I. Kim, J.-H. Shim, and W.-S. Jung, Journal of Alloys and Compounds 658, 210 (2016).
K. H. A. Al-Hatab, M. A. Al-Bukhaiti, U. Krupp, and M. Kantehm, Oxidation of Metals 75, 209 (2011).
D. M. England and A. V. Virkar, Journal of the Electrochemical Society 146, 3196 (1999).
J.-H. Kim, B. K. Kim, D. I. Kim, P. P. Choi, D. Raabe, and K. W. Yi, Corrosion Science 96, 52 (2015).
C. Ostwald and H. J. Grabke, Corrosion Science 46, 1113 (2004).
A. C. S. Sabioni, A. M. Huntz, L. C. Borges, and F. Jomard, Philosophical Magazine 87, 1921 (2007).
A. C. S. Sabioni, A. M. Huntz, F. Silva, and F. Jomard, Materials Science and Engineering A 392, 254 (2005).
A. C. S. Sabioni, A. M. Huntz, J. N. V. Souza, F. Jomard, and M. D. Martins, Philosophical Magazine 88, 391 (2008).
D. Kim, C. Jang, and W. S. Ryu, Oxidation of Metals 71, 271 (2009).
D. L. Douglass and J. S. Armijo, Oxidation of Metals 2, 207 (1970).
A. Ul-Hamid, Anti-Corrosion Methods and Materials 51, 216 (2004).
S. Pedrazzini, E. S. Kiseeva, R. Escoube, et al., Oxidation of Metals 89, 375 (2018).
J. Litz, A. Rahmel, M. Schorr, and J. Weiss, Oxidation of Metals 32, 167–184 (1989).
E. Schmucker, C. Petitjean, L. Martinelli, P. J. Panteix, S. Ben Lagha, and M. Vilasi, Corrosion Science 111, 474 (2016).
T. Connolley, P. A. S. Reed, and M. J. Starink, Materials Science and Engineering A 340, 139 (2003).
H. Nakajima, JOM The Journal of the Minerals, Metals & Materials Series (TMS) 49, 15 (1997).
E. Schmucker, C. Petitjean, L. Martinelli, P.-J. Panteix, B. Lagha, and M. Vilasi, Corrosion Science 111, 467 (2016).
B. Jönsson and A. Westerlund, Oxidation of Metals 88, 315 (2017).
D. Kim, D. Kim, H. J. Lee, C. Jang, and D. J. Yoon, Journal of Nuclear Materials 441, 612 (2013).
Acknowledgements
The authors wish to thank Axel Kranzmann for helpful discussions and hints.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nofz, M., Dörfel, I., Sojref, R. et al. Microstructure of Bare and Sol–Gel Alumina-Coated Nickel-Base Alloy Inconel 625 After Long-Term Oxidation at 900 °C. Oxid Met 91, 395–416 (2019). https://doi.org/10.1007/s11085-019-09888-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09888-z