Abstract
Heat exchange surfaces in biomass- and waste-fired boilers are exposed to corrosive species due to the considerable amounts of alkali chlorides and PbCl2 which are released during combustion. The corrosivity of alkali chlorides toward superheater alloys exposed at high temperature has been studied extensively. However, at lower material temperatures, i.e., at waterwall conditions, considerably less research has been performed. In order to investigate the effect of small amounts of KCl and PbCl2 during the initial stages of the corrosion attack, a Fe–2.25Cr–1Mo steel was exposed for 24 h in an atmospheres consisting of O2 + H2O + N2 at 400 °C. Both KCl and PbCl2 resulted in an increased corrosion rate compared to the reference. The aim of the present paper is to investigate the influence of KCl and PbCl2 on the initial oxidation of a Fe–2.25Cr–1Mo steel. The work involves a detailed microstructural investigation as well as thermodynamic equilibrium calculations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fireside environment in biomass- and waste-fired boilers is characterized by high levels of alkali and HCl, which both are believed to cause elevated corrosion rates. To increase the power production from biomass- and waste-fired boilers, it is necessary to increase the steam data of these plants. This increase in steam data implies not only an increase in the material temperature of the superheaters, but also an increase in material temperature of the waterwalls. Low-alloyed steels are often used as waterwall material due to its low cost and mechanical properties. These steels form an oxide scale of Fe2O3 and Fe3O4 which offer sufficient protection in mildly corrosive environments at moderate temperatures (<500 °C) [1]. At higher temperatures or if the corrosivity of the environment increases, other steel grades and/or coatings are used. In comparison with corrosion of superheaters, the research focused on waterwalls has been scarce. Regarding the corrosion attack of superheaters, where the material temperature is higher, it has been shown by several studies that alkali chlorides are one of the main contributors to the corrosion issues [2–9]. Recent studies have shown that KCl and PbCl2 also accelerates the corrosion rate at the relatively moderate temperatures that exist at the waterwalls [10–13].
Experimental Procedures
Sample Preparation and Analysis
The alloy studied is a Fe–2.25Cr–1Mo steel (also referred to as T22 or 10CrMo910) (see Table 1) for chemical composition. The geometrical area of the sample was 5.56 cm2. A hole (ø = 1.5 mm) was drilled for ease of handling. Before exposure, the samples were ground to 320 grit on SiC and polished with three diamond suspensions 9, 3, and 1 µm until the surface appeared mirror-like. The polished samples were degreased and cleaned in acetone and ethanol using ultrasonic agitation.
A tube furnace was used for the exposure, and the temperature was kept at 400 ± 1 °C. The reference atmosphere consisted of N2 with 5% O2 and 20% H2O (g). The salt was deposited on the samples by means of spraying a solution of the desired salt in a 20:80 water/ethanol mixture and subsequently drying with cool air. The amount of salt was applied to result in a weight of 0.1 mg/cm2 KCl on the surface. In the case of PbCl2, 0.185 mg/cm2 was applied to get equal amounts of Cl in the two cases. Reference samples, without any KCl or PbCl2, were also investigated. The samples were positioned parallel to the direction of the gas flow, in the center of the furnace. All parts of the system were kept above the dew point of water corresponding to 20% H2O vapor to prevent condensation. Prior to and after exposure, the samples were stored in desiccators over a drying agent of P2O5(s).
Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX)
The samples were characterized using a FEI Quanta ESEM 200, equipped with a field emission gun. The microscope was operated in high vacuum mode. The instrument has an Oxford Inca energy-dispersive X-ray (EDX) system for chemical analysis. The acceleration voltage was between 8 and 15 kV for imaging and 15 kV for EDX analysis. Backscattered electrons were used for imaging.
Broad Ion Beam (BIB)
A LeicaTIC3X was used for cross section preparation of exposed samples. A 0.5-mm-thick silicon wafer was glued to the sample with Loctite® 415. The sample was cut with a low-speed saw without lubrication. The three ion guns were operated at 6.5 kV for a duration of 6 h. The attained cross section is approximately 500–1000 µm in width.
X-ray Diffraction (XRD)
A Siemens D5000 powder diffractometer was used to determine crystalline phases present after exposure. The instrument was equipped with a grazing incidence beam attachment together with a Göbel mirror. Cu Ka radiation (λ = 1.52418 Å) was used with an angle of incidence 0.5°–2°. The measurement range was 10° < 2θ < 65°.
Phase diagrams
The thermodynamic calculations were performed using the software FactSage 6.4 and databases: Fact53, ELEM and BINS.
Results and Discussion
Gravimetry
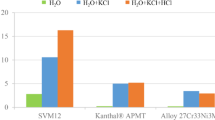
After 24 h of exposure at 400 °C without chloride salts, the mass gain of the Fe–2.25Cr–1Mo is relatively low (see Fig. 1). The presence of KCl increases the corrosion rate substantially with a mass gain of approximately 0.9 mg/cm2 after 24 h. In the presence of PbCl2, the mass gain (0.62 mg/cm2) after 24 h is high compared to the reference, but lower than that of the samples exposed in the presence of KCl. This indicates that in these exposure conditions, KCl is more corrosive of the two salts.
Morphology and Surface Analysis
In the reference case without the presence of chloride salts, the steel has formed a homogenous and smooth oxide all over the surface (see Fig. 2a). In the presence of KCl, the corrosion morphology of Fe–2.25Cr–1Mo is drastically altered (see Fig. 2b). The morphology is characterized by an undulating oxide with KCl particles being partly or fully overgrown by the oxide. Furthermore, the corrosion attack does not seem to be localized to the vicinity of KCl particles. The severe attack of the area between particles, i.e., no KCl present prior to exposure, indicates a fast transport of ions over the surface. The KCl-exposed sample also shows signs of crack formation. The corresponding exposure of Fe–2.25Cr–1Mo in the presence of PbCl2 results in a quite different morphology compared to KCl (see Fig. 2c). The deposited PbCl2 particles have after 24-h exposure completely reacted into PbO, which is seen as the bright areas in the image. This is in line with the XRD analysis in Table 2 showing no diffraction of PbCl2, whereas PbO is detected. Contrary to the samples exposed in the presence of KCl, the corrosion attack of the samples with PbCl2 is more localized to the vicinity of the Pb-containing structures. The XRD analysis in Table 2 shows that KCl is still present after exposure. Furthermore, Fe2O3 and Fe3O4 were detected on all samples.
Scale Microstructure After Exposure
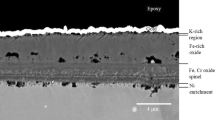
In the reference case, a thin and well-adhered oxide has formed on the Fe–2.25Cr–1Mo (see the BIB cross section in Fig. 3). The oxide scale consists of a Fe-rich outward-growing part and an inward-growing part that contains Cr and Fe. The total thickness of the scale is approximately 1 µm. As indicated by the XRD analysis, the scale is a mixture of M3O4 and Fe2O3. The Cr marks the initial surface before exposure, since the diffusion of Cr in M3O4-type oxides is considerably slower compared to Fe [14]. The Cr at the surface is tied up in the M3O4, thereby marking the interface between outward-/inward-growing oxide.
The undulating morphology seen in plan view SEM analysis for the KCl-exposed sample in Fig. 2b is confirmed by the BIB cross section in Fig. 4. The sample exposed to KCl exhibits a layered oxide with numerous voids and poorly attached oxide scales. This type of morphology has recently been reported to be due to KCl particles being consumed, leaving a void behind [15]. The corrosion attack in KCl is rather general, and there is no clear correlation between the original location of the KCl particles and the corrosion attack. The uniform attack indicates a fast surface transport. This may be explained by the formation of a thin liquid film of an eutectic melt in the FeCl2–KCl system, as proposed by [15]. In the present study, there are not any signs of this thin film (see Fig. 4). However, due to the poor resolution of the SEM/EDX analysis and the progress of the corrosion attack, it is not expected to detect this liquid film. Further analysis with, for example, TEM would be needed. In contrast, traces of subscale metal chlorides can be seen in the EDX analysis in Fig. 4. The porous and non-adherent oxide scales formed on Fe–2.25Cr–1Mo are expected to possess rather poor protection for further oxidation.
According to gravimetry, the mass gain of the sample exposed to PbCl2 was only about 70% of the mass gain of the KCl-exposed sample, indicating a slightly less corrosive environment. The BIB cross section of Fe–2.25Cr–1Mo exposed in the presence of PbCl2 is shown in Fig. 5. Compared to the KCl-exposed samples, the appearance of the oxide scale is significantly different. Instead of a widespread attack all over the surface, as in the case with KCl, the presence of PbCl2 induces a rather localized attack. The thickest oxide scale is located underneath and in the vicinity of an original salt particle. The cross section shows an area of the sample where the PbCl2 was located prior to exposure. The original PbCl2 particle has reacted into PbO, as can be seen in the Pb and Cl EDX maps where no correlation is detected. The fast conversion of PbCl2 into PbO results in a rapid release of chlorine which has penetrated the scale and is located as metal chlorides at the steel/scale interface. Another reason for the localized attack may be that the eutectic temperature for the FeCl2–PbCl2 system (450 °C) is above the exposure temperature which would prevent spreading of the salt (see Fig. 6). Despite the comparably greater presence of subscale metal chlorides in the PbCl2 exposure compared to the KCl exposure, the oxide scale on the PbCl2 seems to be more adherent and dense. However, this may be an effect of the different progress of the corrosion attack in the two cases; for KCl, the subscale metal chlorides may have been evaporated, causing the porous scale, whereas for PbCl2, the corrosion attack has not yet advanced to this stage. The fast reaction of PbCl2 to PbO may in addition explain the less severe attack in the presence of PbCl2 compared to Fe–2.25Cr–1Mo exposed in the presence of KCl, where KCl remains on the surface for a longer duration, see reactions 1–4.
p(HCl) = 1.1*10−10 atm at 400 °C calculated with FactSage
p(HCl) = 1.5*10−5 atm at 400 °C calculated with FactSage
p(KCl) = 5.4*10−10 atm at 400 °C, calculated with FactSage
p(PbCl2) = 5.7*10−6 atm at 400 °C, calculated with FactSage
Conclusions
The initiation of the corrosion attack of Fe–2.25Cr–1Mo is very rapid under the furnace waterwall conditions in this study.
-
The presence of KCl and PbCl2 both increased the corrosion rate on Fe–2.25Cr–1Mo compared to the reference (in the absence of salt) after 24 h of exposure at 400 °C.
-
Exposure of Fe–2.25Cr–1Mo in the presence of KCl resulted in a widespread corrosion attack, which partially can be attributed to the presence of a eutectic composition in the FeCl2–KCl system, resulting in a concentration gradient which spreads KCl over the surface.
-
Exposure of Fe–2.25Cr–1Mo in the presence of PbCl2 resulted in a localized corrosion attack due to fast decomposition of PbCl2 with a simultaneous release of chlorine. The localized nature of the attack can be attributed to the fast decomposition of PbCl2 into PbO, and also the exposure temperature is below the eutectic temperature in the FeCl2–PbCl2 system which leads to a local release of chlorine.
References
P. Kofstad, High temperature corrosion, (Elsevier Applied Science, London, 1988).
J. Lehmusto, B. J. Skrifvars, P. Yrjas and M. Hupa, Comparison of potassium chloride and potassium carbonate with respect to their tendency to cause high temperature corrosion of stainless 304L steel. Fuel Processing Technology 105, 98 (2013).
J. Pettersson, C. Pettersson, H. Asteman, J. E. Svensson and L. G. Johansson, A pilot plant study of the effect of alkali salts on initial stages of the high temperature corrosion of alloy 304L. Material Science Forum 461–464, 965 (2004).
J. Pettersson, H. Asteman, J.-E. Svensson and L.-G. Johansson, KCl induced corrosion of a 304-type austenitic stainless steel at 600 °C; the role of potassium. Oxidation of Metals 64, 23 (2005).
S. Karlsson, J. Pettersson, L.-G. Johansson and J.-E. Svensson, Alkali induced high temperature corrosion of stainless steel: the influence of NaCl, KCl and CaCl2. Oxidation of Metals 78, 83 (2012).
J. Pettersson, C. Pettersson, N. Folkeson, L.-G. Johansson, E. Skog and J.-E. Svensson, The influence of sulphur additions on the corrosive environment in a waste-fired CFB boiler. Materials Science Forum 522–523, 563 (2006).
H. P. Nielsen, F. J. Frandsen, K. Dam-Johansen and L. L. Baxter, The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Progress in Energy and Combustion Science 26, 283 (2000).
S. C. Cha and M. Spiegel, Local reactions of KCl particles with iron, nickel and chromium surfaces. Materials and Corrosion 57, 159 (2006).
A. Zahs, M. Spiegel and H. J. Grabke, Chloridation and oxidation of iron, chromium, nickel and their alloys in chloridizing and oxidizing atmospheres at 400–700 °C. Corrosion Science 42, 1093 (2000).
N. Folkeson, T. Jonsson, M. Halvarsson, L.-G. Johansson, J.-E. Svensson, The influence of small amounts of KCl(s) on the high temperature corrosion of a Fe-2.25Cr-1Mo steel at 400 and 500°C. Materials and Corrosion 62, 606 (2011).
S. Enestam, D. Bankiewicz, J. Tuiremo, K. Makela and M. Hupa, Are NaCl and KCl equally corrosive on superheater materials of steam boilers? Fuel 104, 294 (2013).
D. Bankiewicz, P. Yrjas, D. Lindberg and M. Hupa, Determination of the corrosivity of Pb-containing salt mixtures. Corrosion Science 66, 225 (2013).
D. Bankiewicz, S. Enestam, P. Yrjas and M. Hupa, Experimental studies of Zn and Pb induced high temperature corrosion of two commercial boiler steels. Fuel Processing Technology 105, 89 (2013).
J. Topfer, S. Aggarwal and R. Dieckmann, Point-defects and cation tracer diffusion in (CrxFe1-x)(3-δ)O-4 spinels. Solid State Ionics 81, 251 (1995).
T. Jonsson, T. Folkeson, J.-E. Svensson and L.-G. Johansson, An ESEM in situ investigation of initial stages of the KCl induced high temperature corrosion of a Fe-2.25Cr-1Mo steel at 400°C. Corrosion Science 53, 2233 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Larsson, E., Gruber, H., Hellström, K. et al. A Comparative Study of the Initial Corrosion of KCl and PbCl2 on a Low-Alloyed Steel. Oxid Met 87, 779–787 (2017). https://doi.org/10.1007/s11085-017-9765-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-017-9765-x