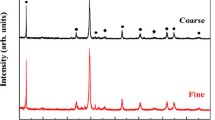
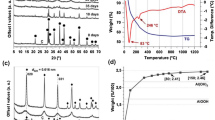
Abstract
The reaction behavior of α-Si3N4 powder at 1100–1500 °C for 10 h under different oxidizing conditions was investigated using thermogravimetric analysis. The phase constitution and microstructure were characterized using X-ray diffraction, optical microscope, scanning electron microscopy, transmission electron microscope and fourier transformation infrared spectroscopy. The results showed that the reaction behavior of α-Si3N4 powder varies depending on temperature and the oxidizing atmosphere. At 1100–1200 °C, water vapor enhanced the reaction due to its specifically inward oxidation. Above 1300 °C, H2O further reacted with SiO2 to form silicon tetrahydroxide, which caused the reaction behavior to follow the paralinear rate law.










Similar content being viewed by others
References
T. Otani and M. Hirata, Thin Solid Films 442, 2003 (44).
S. Ogata, N. Hirosaki, C. Kocer and Y. Shibutani, Acta Materialia 52, 2004 (233).
V. Balaji, A. N. Tiwari and R. K. Goyal, Polymer Engineering & Science 51, 2011 (509).
K. Anzai and H. Hashimoto, Journal of Materials Science 12, 1977 (2351).
K. Komeya, American Ceramic Society Bulletin 63, 1984 (1158).
W. A. Sanders and D. W. Mieskowski, American Ceramic Society Bulletin 64, 1985 (304).
L. Chuck, S. M. Goodrich, N. L. Hecht and D. E. McCullum, Ceramic Engineering and Science Proceedings 11, 1990 (1007).
D. S. Fox, E. J. Opila, Q. G. N. Nguyen, D. L. Humphrey and S. M. Lewton, Journal of the American Ceramic Society 86, 2003 (1256).
A. F. Hampton and H. C. Graham, Oxidation of Metals 10, 1976 (239).
T. Suetsuna and T. Ohji, Journal of the American Ceramic Society 88, 2005 (1139).
R. M. Horton, Journal of the American Ceramic Society 52, 1969 (121).
M. Maeda, K. Nakamura and M. Yamada, Journal of Materials Science 25, 1990 (3790).
Y. G. Gogotsi and F. Porz, Corrosion Science 33, 1992 (627).
Z. Zheng, R. E. Tressler and K. E. Spear, Corrosion Science 33, 1992 (569).
G. Blugan, D. Wittig and J. Kuebler, Corrosion Science 51, 2009 (547).
D. Galusková, M. Kašiarová, M. Hnatko, D. Galusek, J. Dusza and P. Šajgalík, Corrosion Science 85, 2014 (94).
E. J. Opila, D. S. Fox and N. S. Jacobson, Journal of the American Ceramic Society 80, 1997 (1009).
N. S. Jacobson, E. J. Opila, D. L. Myers and E. H. Copland, The Journal of Chemical Thermodynamics 37, 2005 (1130).
A. V. Plyasunov, Geochimica et Cosmochimica Acta 75, 2011 (3853).
A. Hashimoto, Geochimica et Cosmochimica Acta 56, 1992 (511).
J. L. Smialek, R. C. Robinson, E. J. Opila, D. S. Fox and N. S. Jacobson, Advanced Composite Materials 8, 1999 (33).
X. M. Hou, K. C. Chou, X. J. Hu and H. L. Zhao, Journal of Alloys and Compounds 459, 2008 (123).
X. M. Hou and K. C. Chou, Corrosion Science 50, 2008 (2367).
C. S. Tedmon, Journal of the Electrochemical Society 69, 1966 (674).
E. J. Opila and N. S. Jacobson, Oxidation of Metals 44, 1995 (527).
E. J. Opila and R. E. Hann, Journal of the American Ceramic Society 80, 1997 (197).
X. M. Hou, Z. Y. Yu, Z. Y. Chen, K. C. Chou and B. J. Zhao, Journal of the American Ceramic Society 96, 2013 (1877).
E. J. Opila, Journal of the American Ceramic Society 77, 1994 (730).
W. S. Liao, C. H. Lin and S. C. Lee, Applied Physics Letters 65, 1994 (2229).
M. Fukushima, Y. Zhou, Y. I. Yoshizawa and K. Hirao, Journal of the European Ceramic Society 28, 2008 (1043).
Z. Hong, L. Cheng, L. Zhang and Y. Wang, Journal of the American Ceramic Society 92, 2009 (193).
Acknowledgments
The authors express their appreciation to the National Science-technology Support Plan Projects (Grant 2013BAF09B01), National Nature Science Foundation of China (51104012), New Century Excellent Talents in University (NECT-12-0779) and the Fundamental Research Funds for the Central Universities (FRF-TP-14-113A2) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, E.H., Dong, H., Chen, J.H. et al. The Reaction Behavior of α-Si3N4 Powder at 1100–1500 °C Under Different Oxidizing Conditions. Oxid Met 84, 169–184 (2015). https://doi.org/10.1007/s11085-015-9549-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-015-9549-0