Abstract
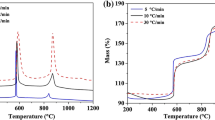
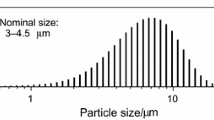
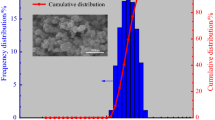
Non-isothermal kinetic analysis of oxidation of aluminum powder particles (100–200 μm) was investigated by simultaneous thermogravimetry (TG) and differential thermal analysis under linear temperature programming (ranging from 25 to 1,400 °C) at different heating rates (10, 20 and 30 °C/min). In addition, the rate of oxidation reaction (rate of thermogravimetry; RTG) was obtained by the RTG curves. It was found that the oxidation of aluminum powders took place over several stages and the complete oxidation process did not occur even up to 1,400 °C. Among different stages, the temperature ranging from 1,000 to 1,150 °C was identified as the main stage for oxidation process. Hence, kinetic analysis of non-isothermal was determined to be carried out in this region only. Therefore, non-isothermal kinetic analysis of oxidation of pure aluminum powder particles was performed using isoconversional methods (Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose, Friedman ), Markworth and Coats–Redfern methods. Also, the empirical kinetic triplets [E a , A, and f(α)] have been calculated.






Similar content being viewed by others
References
L. T. De Luca, L. Galfetti, F. Severini, L. Meda, G. Marra, A. B. Vorozhtsov, V. S. Sedoi and V. A. Babuk, Combustion, Explosion, and Shock Waves 41, 680 (2005).
L. Galfetti, L. T. De Luca, F. Severini, L. Meda, G. Marra, M. Marchetti, M. Regi and S. Bellucci, Matter 18, 1991 (2006).
E.W. Price, R.K. Sigman, in Progress in Astronautics and Aeronautics: Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics, eds V. Yang, T. Brill, and W. Ren, Vol. 185, Chap 2.18 (AIAA Inc., Reston, VA, 2000), pp. 663–687.
H. Dong and S. Zhumei, Combustion and Flame 105, 428 (1996).
J. V. Khaki, M. Panjehpour, Y. Kashiwaya, K. Ishii and M. S. Bafghi, Steel research international 75, 169 (2004).
M. A. Trunov, M. Schoenitz, X. Zhu and E. L. Dreizin, Combustion and Flame 140, 310 (2005).
V. Kolarik, M. M. Juez-Lorenzo and H. Fietzek, Materials Science Forum 696, 290 (2011).
S. Hasani, M. Panjepour and M. Shamanian, Oxidation of Metals 78, 179 (2012).
N. Eisenreich, H. Fietzek, M. M. Juez-Lorenzo, V. Kolarik, A. Koleczko and V. Weiser, On the mechanism of low temperature oxidation for aluminum particles down to the nano-scale. Propellants, Explosives, Pyrotechnics 29, 137 (2004).
E. M. Fryt, Oxidation of Metals 12, 139 (1978).
P. Kofstad, High Temperature Corrosion, (Elsevier Applied Science, London, 1988).
K. Lawless, Reports on Progress in Physics 37, 231 (1974).
P. Kofstad, Acta Chemica Scandinavica 12, 701 (1958).
J. S. Wolf and J. M. Grochowski, in Stress Effect and Oxidation of Metals, ed. J. V. Cathcart (Metall. Soc. AIME, New York, 1975) p. 274.
A. J. Markworth, Metallurgical and Materials Transactions A 8, 2014 (1977).
A. J. Markworth, Metallurgical and Materials Transactions A 10, 377 (1978).
Z. Liu and W. Gao, High Temperature Material Processes 17, 231 (1998).
C. S. Nordahl and G. L. Messing, Thermochimica Acta 318, 187 (1998).
M. Schoenitz, B. Patel, O. Agboh and E. L. Dreizin, Thermochimica Acta 507–508, 115 (2010).
H. Tao, Journal de Physique IV France 12, 105 (2002).
T. A. Roberts, R. L. Burton and H. Krier, Combustion and Flame 92, 125 (1993).
V. I. Kiselev and B. M. Leipinskikh, Kinetics of oxidation of molten aluminum, Report VINITI 542-74, Inst. Metall. Sverdlovsk, USSR (1974) (in Russian).
V. P. Elytin, B. S. Mitin, and V. V. Samotekin, Izvestiya Akademii Nauk SSR, Metally 3, 227 (1971) (in Russian).
M. W. Beckstead, A summary of aluminum combustion, Paper presented at the RTO/VKI Special Course on “Internal Aerodynamics in Solid Rocket Propulsion”, held in Rhode-Saint-Genèse, Belgium, 27–31 May 2002 (RTO-EN-023, 2002).
A. L. Kuhl and V. M. Boiko, Ignition of Aluminum Particles and Clouds, 41st ICT Conference Karlsruhe, Germany June 29 (2010) pp. 1–11.
R. F. Speyer, Thermal Analysis of Materials, (Marcel Dekker Inc., New York, 1993).
M. Schoenitz, C. M. Chen, X. Zhu, and E. L. Dreizin, in Energetic Materials. Characterization, Modeling and Validation, 40th International Conference of ICT, Karlsruhe, Germany (Vol. 34, 2009) pp. 1–11.
M. E. Brown, M. Maciejewski, S. Vyazovkin, R. Nomen, J. Sempere, A. Burnham, J. Opfermann, R. Strey, H. L. Anderson, A. Kemmler, R. Keuleers, J. Janssens, H. O. Desseyn, C. R. Li, T. B. Tang, B. Roduit, J. Malek and T. Mitsuhashi, Thermochimica Acta 335, 125 (2000).
M. Maciejewski, Thermochimica Acta 335, 145 (2000).
S. Vyazovkin, Thermochimica Acta 335, 155 (2000).
A. K. Burnham, Thermochimica Acta 335, 165 (2000).
B. Roduit, Thermochimica Acta 335, 171 (2000).
H. E. Kissinger, Analytical Chemistry 29, 1702 (1957).
T. Akahira and T. Sunose, Trans. Joint Convention of Four Electrical Institutes, Paper No. 246, 1969 Research Report. Chiba Institute of Technology Sci. Technol. 16, 22 (1969).
J. H. Flynn and L. A. Wall, Polymer Letters 4, 323 (1966).
T. Ozawa, Bulletin of the Chemical Society of Japan 38, 1881 (1965).
H. Friedman, Journal of Polymer Science Part C 6, 183 (1964).
S. Vyazovkin and A. I. Lesnikovich, Thermochimica Acta 165, 273 (1990).
S. Vyazovkin and N. Sbirrazzuoli, Macromolecular Rapid Communications 27, 1515 (2006).
P. Budrugeac, Polymer Degradation and Stability 89, 265 (2005).
A. W. Coats and J. P. Redfern, Nature 201, 68 (1964).
S. Li, J. He, P. H. Yu and M. K. Cheung, Journal of Applied Polymer Science 89, 1530 (2003).
S. Vyazovkin and C. A. Wight, Thermochimica Acta 340–341, 53 (1999).
C. Doyle, Journal of Applied Polymer Science 5, 285 (1961).
P. Budrugeac, E. Segal, L. A. Perez-Maqueda and J. M. Criado, Polymer Degradation and Stability 84, 311 (2004).
P. J. Haines, Principles of Thermal Analysis and Calorimetry, (Royal Society of Chemistry, Cambridge, UK, 2002).
A. Khawam and D. R. Flanagan, Thermochimica Acta 436, 101 (2005).
S. Vyazovkin and C. A. Wight, The Journal of Physical Chemistry A 101, 8279 (1997).
M. Erceg, T. Kovacic and S. Perinovic, Thermochimica Acta 476, 44 (2008).
X. Zhu, M. Schoenitz and E. L. Dreizin, The Journal of Physical Chemistry C 113, 6768 (2009).
J. Bouillard, A. Vignes, O. Dufaud, L. Perrin and D. Thomas, Journal of Hazardous Materials 181, 873 (2010).
P. Budrugeac and E. Segal, The International Journal of Chemical Kinetics 33, 564 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasani, S., Panjepour, M. & Shamanian, M. Non-Isothermal Kinetic Analysis of Oxidation of Pure Aluminum Powder Particles. Oxid Met 81, 299–313 (2014). https://doi.org/10.1007/s11085-013-9413-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-013-9413-z