Abstract
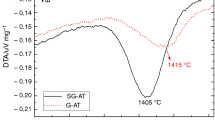
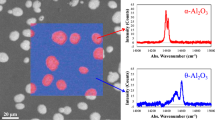
A novel kinetics analysis method to quantifying the extent of metastable → stable Al2O3 phase-transformation in thermally-grown alumina scales is presented. This analysis involved assessing thermogravimetric data and the time dependence of the associated value of the instantaneous time exponent, n i. It was found that if there is θ → α transformation, the n i-value curve characteristically decreases to a minimum and then increases. The time corresponding to the minimum was defined as a transition time, t tr . It was found that after this t tr , the α phase was significantly established as a continuous layer and the overall scaling kinetics were greatly reduced. Furthermore, using a kinetics scenario for the θ and α co-formation, measured weight-gain kinetics could be well simulated from the initial co-formation stage to the steady-state single-α-phase-growing stage. Finally, it is shown that the transformation kinetics in the lateral direction can also be determined by this kinetics analysis method.

















Similar content being viewed by others
References
P. T. Mosely, K. R. Hyde, B. A. Bellany and G. Tappin, Corrosion Science 24, 547 (1984).
I. Levin and D. Brandon, Journal of the American Ceramic Society 81, (8), 1995 (1998).
A. Andoh, S. Taniguchi and T. Shibata, Materials Science Forum 301, 369 (2001).
J. Dokychak and M. Ruhle, Oxidation of Metals 32, 431 (1989).
K. M. N. Prasanna, A. S. Khanna, Ramesh. Chandra and W. J. Quadakkers, Oxidation of Metals 46, 465 (1996).
T. F. An, H. R. Guan, X. F. Sun and Z. Q. Hu, Oxidation of Metals 54, 301 (2000).
P. Burtin, J. P. Brunelle, M. Pijolat and M. Soustelle, Applied Catalysis 34, 225 (1987).
B. W. Veal, A. P. Paulikas and R. C. Birtcher, Applied Physics Letters 89, 161916 (2006).
M. W. Brumm and H. J. Grabke, Corrosion Science 33, 1677 (1992).
D. M. Lipkin, D. R. Clarke, M. Hollatz, M. Bobeth and W. Pompe, Corrosion Science 39, 231 (1997).
M. I. F. Macedo, C. A. Bertran and C. C. Osawa, Journal of the Materials Science 42, 2830 (2007).
D. M. Lipkin, H. Schaffer, F. Adar and D. R. Clarke, Applied Physics Letter 70, 2550 (1997).
D. M. Lipkin and D. R. Clarke, Oxidation of Metals 45, 267 (1996).
X. Peng, D. R. Clarke and F. Wang, Oxidation of Metals 60, 225 (2003).
B. A. Pint, M. Treska and L. W. Hobbs, Oxidation of Metals 47, 1 (1997).
B. Kampfe, P. Patzelt and C. G. Nestler, Kristall and Technik 14, 187 (1979).
R. B. Bagwell, G. R. Messing and P. L. Howell, Journal of the. Materials Science 36, 1833 (2001).
P. Y. Hou, A. P. Paulikasb and B. W. Veal, Materials at High Temperatures 22, (3/4), 535 (2005).
D. M. Lipkin and H. Schaffer, Applied Physics Letters 70, 2550 (1997).
W. Zhao, Ph.D Dissertation, University of Pittsburgh, (2012).
B. Pieraggi, Oxidation of Metals 27, 177 (1986).
J. W. Christian, The Theory of Transformations in Metals and Alloys, (Pergamon Press, University of Oxford, Oxford, 1965).
D. Naumenko, B. Gleeson, E. Wessel, L. Singheiser and W. J. Quadakkers, Metallurgical and Materials Transactions A 38A, 2974 (2007).
H. Al-Badairy, D. J. Prior and G. J. Tatlock, Materials at High Temperatures 22, 453 (2005).
The Mathworks Inc., MATLAB (Version R2012b) [Computer program].
Acknowledgments
This research is supported by the U.S. Office of Naval Research, award N00014-09-1-1127 and managed by Dr. David Shifler. The authors thank Dr. Thomas Gheno for his constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, W., Li, Z. & Gleeson, B. A New Kinetics-Based Approach to Quantifying the Extent of Metastable → Stable Phase Transformation in Thermally-Grown Al2O3 Scales. Oxid Met 79, 361–381 (2013). https://doi.org/10.1007/s11085-013-9365-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-013-9365-3