Abstract
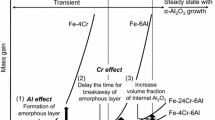
Rapid formation of an α-Al2O3 scale on Fe–50 at.%Al by pure metal thin coatings of Ni, Al, Ti, Cr or Fe was investigated, and the effects of those elements on Al2O3-scale evolution were assessed. The oxidation behavior of samples with and without coatings could be divided into two groups: the samples with/without Ni and Al, and those with Ti, Cr and Fe. The mass gains of samples coated with Al and Ni were almost the same as that of non-coated Fe–50 at.%Al alloy. The mass gains of samples coated with Ti, Cr, and Fe were much lower than that of the Fe–50 at.%Al alloy. A stable α-Al2O3 scale was found to develop from the beginning of oxidation on the samples coated with Ti, Cr and Fe. However metastable θ-Al2O3 remained after long-time oxidation of non-coated and Ni- and Al-coated samples. The direct α-Al2O3 scale formation on the samples with Cr or Fe coatings was speculated to be due to sympathetic nucleation of α-Al2O3 on the surface of Al-supersaturated Fe2O3 for Fe-coated sample, and composition changes from (Cr,Al)2O3 to (Al,Cr)2O3 for the Cr-coated sample. Initial formation of an oxide having a corundum structure was inferred to provide a nucleation site for precipitation of α-Al2O3 without prior formation of a metastable Al2O3 scale.











Similar content being viewed by others
References
T. F. An, H. R. Guan, X. F. Sun, and Z. Q. Hu, Oxidation of Metal 54, 301 (2000).
G. C. Rybicki and J. L. Smialek, Oxidation of Metals 31, 275 (1989).
J. L. Smialek, J. Doychak, and D. J. Gaydosh, Oxidation of Metals 34, 259 (1990).
H. J. Grabke, Intermetallics 7, 115 (1999).
M. W. Brumm and H. J. Grabke, Corrosion Science 33, 1677 (1992).
M. W. Brumm and H. J. Grabke, Corrosion Science 34, 547 (1993).
M. W. Brumm, H. J. Grabke, and B. Wagemann, Corrosion Science 36, 37 (1994).
H. J. Grabke and G. H. Meier, Oxidation of Metals 44, 147 (1995).
H. J. Grabke, D. Wiemer, and H. Viefhaus, Applied Surface Science 47, 243 (1997).
H. J. Grabke, W. M. Brumm, and B. Wagemann, Materials and Corrosion 47, 675 (1996).
C. Houngniou, S. Chevalier, and J. P. Larpin, Oxidation of Metals 65, 409 (2006).
H. Asteman and M. Spiegel, Corrosion Science 50, 1734 (2008).
W. C. Hagel, Corrosion 21, 316 (1965).
F. A. Golightly, G. C. Wood, and F. H. Stott, Oxidation of Metals 14, 217 (1980).
H. E. Kadiri, R. Molins, Y. Bienvenu, and M. F. Horstemeyer, Oxidation of Metals 64, 63 (2005).
F. Liu, H. Gotlind, J. E. Svensson, L. G. Johansson, and M. Halvarsson, Corrosion Science 50, 2272 (2008).
H. Josefsson, F. Liu, J. E. Svensson, M. Halvarsson, and L. G. Johansson, Materials and Corrosion 56, 801 (2005).
A. Andoh, S. Taniguchi, and T. Shibata, Materials Science Forum 369–372, 303 (2001).
Z. Liu and W. Gao, Oxidation of Metals 54, 189 (2000).
P. Burtin, J. P. Brunelle, M. Pijolat, and M. Soustelle, Applied Catalysis 34, 225 (1987).
B. A. Pint, M. Treska, and L. W. Hobbs, Oxidation of Metals 47, 1 (1997).
Y. Kitajima, S. Hayashi, S. Ukai, and T. Narita, Materials Science Forum 595–598, 1013 (2008).
R. Chegroune, E. Salhi, A. Crisci, Y. Wouters, and A. Galerie, Oxidation of Metals 70, 331 (2008).
O. Kubaschewski and R. Schmid-Fetzer, in Ternary Alloys: A Comprehensive Compendium of Evaluated Constitutional Data and Phase Diagrams, eds G. Petzow and G. Effenberg, Vol. 5 (VCH, Cambridge, 1992), p. 325 (Fe2O3–Al2O3 system).
H. I. Aaronson, G. Spanos, R. A. Masamura, R. G. Vardiman, D. W. Moon, E. S. K. Menon, and M. G. Hall, Materials Science and Engineering B32, 107 (1995).
E. N. Bunting, Bureau of Standards. Journal of research 6, 948 (1931).
M. A. Afifi, M. M. Abdel-Aziz, I. S. Yahia, M. Fadel, and L. A. Wahab, Journal of Alloys and Compounds 455, 92 (2008).
Acknowledgments
This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Exploratory Research, 20656115, (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitajima, Y., Hayashi, S., Nishimoto, T. et al. Rapid Formation of α-Al2O3 Scale on an Fe–Al Alloy by Pure-Metal Coatings at 900 °C. Oxid Met 73, 375–388 (2010). https://doi.org/10.1007/s11085-009-9184-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-009-9184-8