Abstract
The synthesis of prebiotic molecules from simple precursors is believed to be a crucial scheme in order to study the origin of life processes. The present study describes the one-pot synthesis of purine and pyrimidine nucleic acid bases in the presence of pre-biologically significant binary metal oxide nanoparticles, metal ferrites, namely NiFe2O4, CoFe2O4, CuFe2O4, ZnFe2O4 and MnFe2O4. The products identified are cytosine, isocytosine, 4(3H)-pyrimidinone, adenine, hypoxanthine and purine. The ability of isocytosine (a constitutional isomer of cytosine) to recognize cytosine and guanine through normal and reversed Watson-Crick pairing respectively, demonstrates an important storyline for the genesis of ancient nucleic acids. The relevance of other synthesized nucleic acid bases with respect to the origin of life is also discussed. The divalent metal ions in iron oxide make it an appropriate catalytic system because it demonstrates excellent catalytic performance for the nucleic acid bases synthesis with significantly high yield, as compared to pure iron oxide and some other minerals like silica, alumina, manganese oxides and double metal cyanide complexes.
Similar content being viewed by others
Introduction
The exact route for the evolution of life on this planet remains a matter of great debate, as current extant life exhibits highly complex pathways, cycles and hypercycles. However, the most logical scenario required the synthesis of polymers, especially information carrying molecules such as RNA and DNA as well as proteins (Walde 2005). Chemical precursors of these molecules, such as hydrogen cyanide (HCN), formamide (HCONH2), cyanoacetylene (C3HN), formic acid (HCOOH) and formaldehyde (HCHO) are thought to have been among the major reactants in prebiotic organic synthesis (Jheeta et al. 2013; Gordon and Sharov 2017). Among these chemical precursors, the roles of HCN and HCONH2 may have been particularly important (Orgel 2004; Delaye and Lazcano 2005; Saladino et al. 2012a). HCONH2 is the simplest amide molecule and can be found in young stellar objects like W33A, the interstellar medium, as well as on comets and the moons of planets such as Saturn’s moon, Titan and Jupiter’s moon, Europa (Schutte et al. 1999; Bockelée-Morvan et al. 2000; Levy et al. 2000; Borucki et al. 2002; Crovisier 2004; Parnell et al. 2006). Apart from the prebiotic availability, some of the key properties of the HCONH2 molecule such as comparative stability, multifaceted reactivity and low volatility make it a good choice of potential substrate for the synthesis of nucleic bases and analogous compounds. (Barks et al. 2010). In addition, the role of the simple triatomic molecule, HCN, in the synthesis of nitrogenous heterocyclic compounds (such as adenine) on primitive Earth has been the subject of extensive research for several decades. (Miller 1955; Abelson 1966). It was Oro and co-workers who first instigated the synthesis of adenine nucleic acid bases from HCN, thus adding an important contribution to the relevance of HCN’s chemistry (Oró 1960; Oró and Kimball 1961; Oró and Kamat 1961). The narrow panel of nucleic bases limited only to purines as well as the thermodynamic instability of HCN were major drawbacks in HCN’s chemistry. In view of this, the option of HCONH2 as a more effective selection of precursors for the synthesis of nucleic acid bases when compared to HCN has been established and is reflected in the research work done by Bredereck et al. 1959; Yamada and Okamoto 1972; Yamada et al. 1978a, b. Metal oxides and minerals may be considered relevant in prebiotic chemistry in the sense that they could mediate the chemistry of HCONH2 by forming a number of prebiotic nitrogenous heterocyclic compounds and thus the scenario could possibly be regarded as a model for the primitive Earth’s environment.
It is generally accepted that the conceptual foundations of the research field of prebiotic chemistry were laid by Oparin and Haldane in the 1920s and subsequently Bernal and (among others) Cairns-Smith (Bernal 1951; Cairns-Smith 1992). Saladino et al. (2001) further developed experimentation through the analysis of the simple catalytic effect of minerals in the thermal condensation process of HCONH2. These minerals or oxides act typically as a heterogeneous catalyst in HCONH2 condensation reactions. Their ability resides in the fact that they can protect the freshly synthesized biologically relevant molecules from both chemical and photochemical damage. (Scappini et al. 2004; Gallori et al. 2006). Moreover, the degradation kinetics of HCONH2 compared to other compounds of prebiotic importance is believed to be controlled by mineral surfaces (Saladino et al. 2008). A large panel of nucleic acid bases, both purine and pyrimidine and their analogous compounds, along with the HCONH2 degradation products upon the condensation of HCONH2 molecule was reported in the presence of catalysts such as silica, alumina, zeolites, zirconium oxide as well as boron, phosphate, iron-sulphur minerals and finally, clays and interstellar dust particles by Saladino and co-workers (2012b). The Earth’s crust contains metal oxides as an essential component, so the possibility of these compounds acting as a catalyst in respect of HCONH2 chemistry cannot be ruled out. Oxides of iron and manganese have also recently been used in HCONH2 condensation reactions (Shanker et al. 2011; Bhushan et al. 2016a).
In a similar vein, spinel ferrite minerals (having a general formula of AIIB2IIIO4; where A may be Zn, Ni, Co, Cu, Mn, etc., and B = Fe.) are present almost everywhere that ancient sediments occur. These spinels are common non-silicate oxides found in the Earth’s crust and upper mantle (Clarke and Washington 1924; Reichmann and Jacobsen 2006). Mineral phases such as trevorite (NiFe2O4) and cuprospinel (CuFe2O4) along with magnesioferrite (MgFe2O4) and chromite (FeCr2O4) were discovered in the Baransky (Iturup Island) and Mutnovskii (southern Kamchatka) hydrothermal systems (Rychagov et al. 1996). Jacobsite mineral (MnFe2O4) was found on manganese-rich rocks from the Ossa–Morena zone (Millan and Velilla 1998), from the Hutter Mine, Pittsylvania County, USA (Beard and Tracy 2002) and from the Central Alps, Switzerland (Brugger and Meisser 2006). In addition, jacobsite rich spinels along with galaxite have been found together in the metamorphosed manganese deposits near Bald Knob, North Carolina, USA (Essene and Peacor 1983). Hydrothermal meta-sedimentary rocks contain jacobsite as reported by Stalder and Rozendaal 2005. A significant amount of ferromanganese nodules (MnFe2O4) has been identified from the Christmas Island region of the Indian Ocean (Exon et al. 2002) and about 2–3 mm sized nodules of the same mineral have also been found on the west coast of the Pacific Ocean (Timofeeva et al. 2014). Cuprospinel has also been found in an ore dump at Baie-Verte, Newfoundland (Nickel 1973). Finally, spinels have been found in several meteorites; for instance, trevorite (NiFe2O4) a spinel ferrite mineral found in the Orgueil Soltmany and Khatyrka meteorites (Hoover 2007; Karwowski 2012; Bindi et al. 2015). Ji et al. (2008) reported a prebiotic chemistry experiment under hydrothermal conditions to synthesize hydrocarbons from CO2 as a starting material on the surface of cobalt ferrite (CoFe2O4). The formation of CoFe2O4 along with other ferrites occurred by the incorporation of transition metals in magnetite minerals which is formed by the serpentinization of peridotites at mid-ocean ridges (Gülaçar and Delaloye 1976; Mével 2003).
With the intention of further evaluating the effect of minerals on the prebiotic chemistry of HCONH2, we focussed our attention on spinel ferrite minerals. These are basically binary oxide minerals such as MnFe2O4, franklinite (ZnFe2O4), NiFe2O4, CuFe2O4, magnetite (FeFe2O4), ulvospinel (TiFe2O4), as well as CoFe2O4. Beside the prebiotic significance of this spinel group of minerals, we chose them because they can be regarded as metal oxide combined with iron oxide and the structural characteristics of spinel ferrite allows us to compare its catalytic activity with respect to pure iron oxide, as well as some previously studied HCONH2 condensation reactions. The principal goal of this article is to address the following question:
-
Does the presence of divalent metal ions in iron oxide minerals improve the synthesis of nucleic acid bases from HCONH2?
Keeping this in mind, a series of spinel ferrite nanoparticles (NiFe2O4, CoFe2O4, CuFe2O4, ZnFe2O4 and MnFe2O4) was synthesized from corresponding metal nitrate salts at high- temperature conditions compatible with a volcanic scenario on primitive Earth and HCONH2 based condensation experiments were performed. The experimental findings reported here, in relation with the latest use of spinel ferrite for the entrapment of ribonucleotides and condensation of amino acids on its surface, shed further light on the role of spinel ferrites in prebiotic chemistry (Iqubal et. al 2015; 2016, 2017).
Experimental Section
Materials and Methods
Nickel (II) nitrate (Ni(NO3)2.6H2O), Cobalt (II) nitrate (Co(NO3)2.6H2O), Copper (II) nitrate (Cu(NO3)2.3H2O), Citric acid (C6H8O7.H2O), and Ethylene glycol (C2H6O2) were provided by E. Merck. Iron (III) nitrate (Fe(NO3)3.9H2O), Manganese (II) nitrate (Mn(NO3)2.4H2O), formamide (> 99.5%) and authentic standard of nucleic acid bases were purchased from Sigma-Aldrich. The reagents were used without further purification. Millipore water was used throughout the studies.
Preparation of Metal Ferrites
The nanosized metal ferrite, AFe2O4, where A = Ni, Cu, Co, and Mn was prepared according to a previously reported procedure (Sharma et al. 2015). In a typical synthesis of nickel ferrite, stoichiometric amounts of Ni(NO3)2.6H2O (0.02 mol, 5.8 g) and Fe(NO3)3.9H2O (0.04 mol, 16.1 g) were prepared separately and then mixed together with constant stirring at 80–90 °C. After complete mixing of the metal salts, citric acid (0.06 mol, 12.6 g) was added, followed by 10 ml of ethylene glycol to the solution. The solution was stirred until gel formation. The obtained gel was subjected to thermal treatment at 400 °C for 2 h in a muffle furnace. The same procedure was also used to synthesize the other ferrites. The synthesized material was characterized by several techniques such as XRD, FE-SEM, TEM, etc. Details of the characterization were found to be the same as reported earlier (Iqubal et. al 2016).
Nucleic Acid Bases Synthesis Protocol from Formamide
In our present work the typical synthesis was as follows: 5.0 ml of neat formamide was taken in a 50 ml round bottom flask, followed by adding 50 mg of a solid metal ferrite catalyst, either nickel ferrite (NiFe2O4), cobalt ferrite (CoFe2O4), copper ferrite (CuFe2O4) zinc ferrite (ZnFe2O4) or manganese ferrite (MnFe2O4). The contents of the flasks were heated at a constant temperature of about 160 °C in an oil bath connected with a condenser apparatus for the duration of between 6 and 48 h. A blank reaction (without adding catalyst) was also performed. The reaction mixture was pipetted out using a micropipette at different time durations between 6 and 48 h, then centrifuged and followed by filtration through a 0.25 μm syringe filter and further progress was monitored by high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry analysis (LC-MS).
High-Performance Liquid Chromatography Analysis
HPLC analysis of the crude reaction mixture was performed using Waters 2489, binary system with UV detection at 260 nm using a Purosphere® RP-18 column (250Χ4.6 mm, 5 μm). Reaction products were eluted with 0.1 M KH2PO4 buffer solution which is acidified with H3PO4 having pH ~4.05. The mobile phase flow for the HPLC analysis was maintained at 0.50 ml/min in isocratic mode.
Liquid Chromatography-Mass Spectrometry Analysis
LC-MS analyses were performed using a Shimadzu LC–MS-8030 (Shimadzu Corporation, Kyoto, Japan), equipped with reversed-phase Shimadzu VP-ODS C18 column (250 × 4.6 mm i.d. 5 μm). The column temperature was set at 35 °C for all analyses. Ionization of analytes was carried out in ESI mode maintaining the condition as: nebulizer gas flow 10 psi, dry gas 10 L min−1, capillary voltage 4000 V, fragmentor voltage 100 V, vaporizer temperature 250 °C. Separation was performed using a gradient solvent system. The mobile phase contains 0.1% acetic acid–water (eluent A) and ACN (eluent B). The gradient was set as follows: 1% B for 0–20 min, and then B was increased to 80% after 20 min and was held for 40 min.
Results
Synthesis of nucleic acid bases from HCONH2 needs heat at a constant temperature in the presence of a suitable catalyst. The temperature up to which HCONH2 can be heated without decomposition is 180 °C and it was taken as a standard upper-temperature limit in view of the boiling point (211 °C) of HCONH2. Thus, an assemblage of conditions for HCONH2 condensation reaction is an appreciable local concentration of HCONH2, a suitable catalytic surface, and temperature levels between 100 and 180 °C (Saladino et al. 2001). We studied the condensation of HCONH2 in the presence of metal ferrite nanoparticles. In the absence of any catalyst (that is, performing a blank reaction) only purine was detected, whilst in the presence of a series of metal ferrites catalysts, HCONH2 afforded various nucleic bases in appreciable yields, namely, cytosine, isocytosine, 4(3H)-pyrimidinone, adenine, hypoxanthine and purine (Table 1). We have used HPLC and LC-MS techniques to identify and quantify the products obtained in our reaction mixture.
High-Performance Liquid Chromatography Analysis
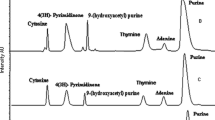
The HPLC chromatogram given in Fig. 1 showed several peaks indicating the detection of various nucleic bases in the reaction mixture. Nucleic bases identified by HPLC were cytosine, isocytosine, 4(3H)-pyrimidinone, adenine, hypoxanthine and purine. The confirmation of the reaction products was achieved with the help of retention time and co-injection methods. Each of the standard compounds having a different set of concentrations was taken in order to generate a standard curve. The detector response was calculated and found to be linear. The peak area comparison of products to that of standards gave the yield of reaction products. The yield of the products is reported in terms of milligrams per gram of HCONH2, in view of the unpredictability of the participation of the number of reactant molecules during the synthesis. It was observed that product formation started within 6 h of the reaction and gradually became constant after 24 h of the reaction (Fig. 2). Our main attention was concentrated on the synthesis and detection of purine and pyrimidine nucleic bases under the experimental conditions, where appreciable yield was noted. All the metal ferrites used here afforded the same purine and pyrimidine nucleic acid bases with different % yields. The blank experiment (without the presence of a catalyst) afforded 40.83 mg/g of purine and, compared to blank reaction results, the formation of purine was found to be the highest (92.78 mg/g) for nickel ferrite, whereas it nearly matched the blank value for manganese ferrite nanoparticles (43.00 mg/g). In addition to purine, an appreciable quantity of 4(3H)-pyrimidinone and adenine was also detected on all the metal ferrite catalysts except CoFe2O4 nanoparticles, where relatively low levels of products were observed when compared to the other catalysts. The other nucleic acid bases namely cytosine, isocytosine, and hypoxanthine were found in the range of 0.06–5.6 mg/g, which is relatively low when compared to the levels of purine, adenine and 4(3H)-pyrimidinone found, which ranged from 2.9 to 92.9 mg/g.
HPLC was done exclusively for quantification purposes. As discussed above, with the help of the standard compounds and co-injection methods we clearly obtained the existence of the mentioned compounds. However, in order to further prove the existence of the synthesized compounds, mass spectra were used for the identification of the ions (compounds) as a confirmatory technique.
Liquid Chromatography-Mass Spectrometry Analysis
The reaction mixture obtained after the condensation of HCONH2 on a metal ferrite surface was then subjected to LC-MS analysis to determine the mass of each of the compounds detected by HPLC. The nucleic bases were separated in an LC-MS and from the mass ion chromatogram, we have established the mass of various nucleic acid bases. Mass spectra of the reaction mixture were analyzed in terms of [M + nH] + ions and gave the ratio results: cytosine m/z 112.0500; 4(3H)-pyrimidinone m/z 97.1000; adenine m/z 136.0500; hypoxanthine m/z 137.1000; and purine m/z 121.1000. Figure 3 shows the corresponding mass spectra of the nucleic acid bases.
Discussion
Several nucleic bases have been made from HCONH2 in the presence of spinel ferrite minerals. The nucleic bases syntheses in the presence of each of the spinel ferrite minerals tested occur under moderate and relatively fast conditions. We chose NiFe2O4, CoFe2O4, CuFe2O4, ZnFe2O4 and MnFe2O4 as representative spinel ferrite compounds. Special emphasis has been given to the ferrites having metals Ni, Co, Cu, Zn and Mn. The possible role of these trace elements in chemical evolution has been increasingly considered important, due to their presence in current biological systems. According to Oskar Baudisch (1943), the transition metal ions present in primeval seas as well as in the Earth’s crust undoubtedly entered into the life cycle ancestrally and thus become a mandatory part of living cells. The precursors of metal enzymes might have played an important role by acting as catalysts for the synthesis of macromolecules of prebiotic significance. Sulfido, carbonyl etc., ligand-bound transition metals such as Fe, Ni, Co etc., can potentially act as catalysts in macromolecule synthesis (Wächtershäuser 1990). Fe, Zn and molybdenum (Mo) are considered to be key elements in all living organisms, whereas Ni, Co, Cu and Mn are essential elements for higher animals’ chemistry only. The elements having multiple oxidation states, such as Fe, Cu, Mo, are of special relevance as they participate in electron transfer catalytic reactions in redox enzymes, whilst elements like Zn and Mn catalyze enzymatic reactions by behaving as Lewis acids (Kobayashi and Ponnamperuma 1985). These metal ions are present in the active sites of several enzymes. For example, Fe, Ni, Co, Cu, Zn and Mn are present as active site metals in the metalloenzymes: ferredoxin, urease, transcarboxylase, cytochrome oxidase, carboxypeptidase A and pyruvate carboxylase respectively (Vallee and Wacker 1976). Metal ferrites can easily be synthesized from the reaction of corresponding metal nitrate salts at high-temperature conditions compatible with the volcanic geological conditions on the early Earth.
where A = Ni2+, Co2+, Cu2+, Zn2+and Mn2+
Spinel ferrite minerals can be treated as divalent metal oxides combined with iron (III) oxides of the type AO.Fe2O3. These particular minerals have the general formula AB2O4 where A = a divalent metal ion eg Ni2+, Co2+, Cu2+, Zn2+, Mn2+ and B = FeIII cation. In the spinel-type structure, there are Td (tetrahedral) and Oh (octahedral) sites that are denoted by A and B while O represents the anionic sites. The unit cell of the spinel ferrite is categorized as a cubic structure containing 8 units of AOFe2O3 and 32 of O2− anions. The partial packing of FeIII and AII cations among the 64Td and 32 Oh sites complete the close face-centred cubic (FCC) skeleton of spinel ferrites (Mehdiye et al. 2008). They have the same crystallographic structure as mineral spinel (e.g. MgAl2O4) and that is the reason they are known as spinel ferrite minerals.
In the HCONH2 condensation reactions, the role of the catalyst is pivotal. Catalysts provide their surfaces for concentrating the HCONH2 molecules, reducing the necessary energy of activation of product formation and further preserving the product from degradation through the surface binding phenomenon. Basically, the HCONH2 guest molecule is positioned on the surface of the catalyst in an ordered structure through the non-covalent interaction taking place between the host (catalyst) and the guest (HCONH2). The well-arranged layout of the guest molecule on the surface of the host along with the non-covalent interactions taking place between them can activate the overall condensation processes. In respect of the present study, the catalytic effect of spinel ferrite minerals in HCONH2 condensation is probably due to the presence of transition metal atoms in their corresponding structures. Demonstrating which parts of the minerals are acting as a catalyst in the reaction system is beyond the scope of this study. However, in accordance with the previous study regarding HCONH2 condensation (Kumar et al. 2014) and in analyzing the catalytic role of spinel ferrite minerals in several heterogeneous catalytic reactions (Viswanathan et al. 1979; Ramankutty and Sugunan 2001; Wang et al. 2012; Kooti and Afshari 2012; Kharisov et al. 2014; Agrawal and Singh 2016), we propose a probable surface interaction scenario involving HCONH2 and the metal ions present in the framework of spinel ferrite nano-particles as given in Fig. 4. The surface distribution of the divalent and trivalent cations in the Td and Oh sites of the spinel ferrite structure primarily direct the catalytic activity of the metal ferrites. It is well established that the octahedral sites of the spinel structure are almost exclusively exposed towards the interacting molecules and that is why octahedral cations in the spinel structure are mainly responsible for showing the catalytic activities towards a heterogeneous catalytic reaction (Jacobs et al. 1994). Moreover, metal ions can interact freely with the reactant molecules because octahedral sites are placed at larger distances. As shown in Fig. 4, in the normal spinel structure, divalent metal ions are completely located in tetrahedral sites where octahedral sites are populated by trivalent iron. On the other hand, in the case of an inverse spinel structure, divalent metal ions are arranged in tetrahedral sites whereas trivalent iron is equally distributed between tetrahedral and octahedral sites. The structure of the spinel is well described by the parameter λ, defined as the fraction of B ions present in tetrahedral sites. The value of λ ranges from 0 for normal spinels to 0.5 for inverse spinels. According to literature (Huheey et al. 2006), all of the spinel minerals tested in the present study are of the inverse type - except zinc ferrite which is a normal spinel. An electrostatic interaction may take place between electropositive metal ions present in the spinel ferrite catalyst and HCONH2 bearing electronegative oxygen atoms. As in the inverse structure, octahedral sites are occupied by divalent metal ions and trivalent iron, so it is likely that these two ions will participate in the interaction with HCONH2 molecule, whereas in normal spinels the octahedral sites are completely occupied by trivalent iron, so here a contribution of divalent metal ion in the interaction process may be treated as negligible.
Concerning the prebiotic significance of HCONH2 products, we are mainly interested in the synthesis and characterization of purine and pyrimidine derivatives. Amongst the nucleic bases that were synthesized, adenine and cytosine are the natural nucleic bases. Although isocytosine is not a natural nucleic base, it still has considerable prebiotic significance; isocytosine, a constitutional isomer of cytosine is capable of identifying cytosine via Normal Watson-Crick (NWC) pairing and it recognizes guanine via Reversed Watson-Crick (RWC) interactions (Zhanpeisov and Leszczynski 1999; Oyelere et al. 2002). The ability of isocytosine to identify cytosine via NWC hydrogen bonding akin to that of cytosine/guanine couple could perhaps imitate guanine as a substitute ancient genetic alphabet. It is noteworthy that recognition of guanine by cytosine through RWC interactions increases the feasibility of hydrogen bonding interactions in substitute pre-ribonucleic acid molecules. (Gupta et al. 2004). Moreover, simple hydrolysis of cytosine to give uracil makes it a potential choice of substrate for the synthesis of uracil. Cytosine can also pair up with adenine analogs through GC/AT transitions in the following replication (Hirao et al. 2002). A considerable amount of pyrimidine nucleic base 4-(3H)-pyrimidinone was obtained in the presence of all of the spinel ferrite minerals. This pyrimidine nucleic base is of significant prebiotic importance, as it is found to be abundant in the Murchison meteorite (Stoks and Schwartz 1982; Botta and Bada 2002). The possible prebiotic role of nucleic bases has been investigated in recent times by researchers. Gounaris and co-workers (Gounaris et al. 2014) shed light on the prebiotic synthesis of the amino acid, alanine, demonstrating that cytosine can reductively aminate the α-keto acid pyruvate into amino acid alanine. The biological importance of hypoxanthine lies in the fact that it is mutagenic by inducing A/T → G/C transitions at the DNA damaged site (Karran and Lindahl 1980; Hill-Perkins et al. 1986). Inherent uniform hydrogen bond patterns in hypoxanthine between the keto and enol forms may make it a substitutive choice of nucleic base for the ancestor of the A-T(U) and G-C base pairs because it can pair up with both T(U) and C by means of simple tautomerism (Shugar and Kierdaszuk 1985). In addition, hypoxanthine is believed to be an important intermediate in the synthesis and degradation of purine nucleotides (Martins et al. 2008).
A comparative study of the mineral in relation to HCONH2 condensation reactions is difficult because the trend in the product formation may depend on several properties of the mineral such as surface area, method of preparation, particle size, structural shape, surface acidity, oxidation state, number of products formed etc. However, a few previous studies have been discussed that can allow us to compare the experimental results reported here. Iron (III) oxide minerals, namely hematite, goethite and akaganeite have been used for the HCONH2 condensation reactions and gave purine, adenine, cytosine, 9-(hydroxyacetyl) purine and 4(3H)-pyrimidinone whilst in the presence of a series of manganese oxides, namely manganosite (MnO), bixbyite (Mn2O3), hausmannite (Mn3O4) and pyrolusite (MnO2), HCONH2 gave the same set of products along with thymine. The dissimilar structural characteristics of iron oxide minerals played an important role during the synthesis of nucleic bases where akaganeite, having a body-centered-cubic arrangement, was found less catalytically active when compared to hexagonally-close-packed structures bearing goethite and hematite. Among the employed manganese oxides in HCONH2 condensation, the lower oxides of manganese were proved to be efficient catalysts that support the catalytic activity of these oxides in early Earth’s reducing atmosphere (Shanker et al. 2011; Bhushan et al. 2016a, b). Surface acidity of montmorillonite clay was portrayed as a key parameter towards the efficient synthesis of nucleic base and their analogous compounds as reported by Saladino et al. 2004. Among the metal ferrites, nickel ferrite (having the largest surface area of 80.64 m2/g) gave the highest yield of nucleic acid bases in the case of adenine, and isocytosine only; manganese ferrite having the lowest surface area (22.97 m2/g), yielded higher levels of cytosine nucleic acid bases compared to nickel ferrite under identical conditions. The surface area of the ferrites is given in Table 2. The yield of the product was in accordance with surface area with respect to purine only, but as we compare this to the yield of other nucleic acid bases, the trend is not followed at all. For instance, the surface area of manganese ferrite (22.97 m2/g) is nearly 2.5 times smaller than the surface area of cobalt ferrite (53.66 m2/g) but the yield of the nucleic acid base 4(3H)-pyrimidinone was found to be almost 11 times higher in the case of the former compound. Yields of other nucleic acid bases such as adenine, cytosine, isocytosine, and 4(3H)-pyrimidinone were also high in the case of manganese ferrite when compared to cobalt ferrite. The correlation between the yield of the products and surface area of the samples suggests that surface area is not a crucial parameter during the condensation of HCONH2 and this experimental observation is in accord with the zirconium oxide mineral catalyzed HCONH2 condensation reaction as performed by Saladino et al. (2010). In contrast, Kumar et al. (2014) reported surface areas to be the dominating parameter in the HCONH2 condensation reaction in the presence of a series of metal(II) octacyanomolybdate complexes. The different behavior in respect to the governing parameters that affects the yield of the products between a metal oxide and double metal cyanide complexes could probably be due to different mineral types. In reality, HCONH2 chemistry is mainly surface chemistry and any parameter that can optimize the interaction between the surface mineral and HCONH2 will optimize (in principle) the yield of the process. It is noteworthy that some minerals can be partially soluble in HCONH2 (e.g. phosphate minerals; Saladino et al. (2006). In these cases, the system is expected to be more complex, forming miscellanea between homogeneous and heterogeneous catalysis. The yield of the reaction products in the presence of spinel ferrite nanoparticles was found to be appreciably higher compared to some previously studied catalytic systems comprising of silica, alumina, iron oxides, manganese oxides and metal(II) octacyanomolybdate complexes (Saladino et al. 2001; Shanker et al. 2011; Kumar et al. 2014; Bhushan et al. 2016a, b). That makes it a unique material for HCONH2 condensation reactions in respect of prebiotic chemistry. It is well established that ferrites present fascinating catalytic activity in comparison to monocomponent metal oxides (Kooti and Afshari 2012). The enhanced catalytic performance of bimetallic ferrite nanoparticles in relation to single component catalytic systems could possibly be due to their unique chemical and physical properties, arising from the synergistic effect between the two metals (Toshima and Yonezawa 1998; Beletskaya and Tyurin 2010; Moghaddam et al. 2014; Roy et al. 2016). Moreover, as discussed earlier the large unit cell of metal ferrite consists of 64 Td and 32 Oh sites. Out of these, only 16 Oh and 8 Td sites are filled by divalent and trivalent cations (Goldman 2006). Thus, a huge portion of interstitial empty sites in the crystal structure of spinel ferrite can act as grooves and could possibly enhance the overall catalytic activity in the condensation process. Complete comparative studies with all the minerals tested for HCONH2 condensation reactions seems unrealistic because a systematic comparison can only be done when very similar sets of products are obtained in each case. That is why we have not considered those previously described syntheses for comparison (Saladino et al. 2003, 2004, 2005, 2006, 2008, 2010, 2011a, b) where a large number of other competitive products were identified compared to the metal ferrite catalyzed HCONH2 condensation experiment.
It is a shared opinion that minerals possessing metal in its reduced state rather than in an oxidized state might have been more efficient in the context of chemical evolution and the origin of life. (Saladino et al. 2008; Bhushan et al. 2011, 2016a, b). The current study deals with the catalytic activity of spinel ferrite minerals where iron is in an oxidized form as Fe3+, along with divalent metal ions A2+ towards nucleic acid base synthesis from HCONH2. The experimental observation revealed that divalent transition metal oxides combined with iron (III) oxides (belonging in a spinel category with oxidized iron) are important for the synthesis of purine and pyrimidine nucleic acid bases from HCONH2. The capability of oxidized iron (Fe3+) and divalent metal ions (M2+) for nucleic acid base synthesis from HCONH2 as reported in this study is in accord with Shanker et al. (2011), where they have used only pure FeIII oxide. The divalent metal ion in iron oxide makes it a novel catalytic system because it demonstrates excellent catalytic performance for nucleic acid base synthesis with significantly high yields when compared to pure iron oxide and some other minerals as discussed above.
Conclusion
The synthesis of a number of purine and pyrimidine nucleic acid bases from the thermal condensation of the prebiotic molecule, HCONH2 in the presence of metal ferrite nanoparticles has been demonstrated. A series of catalysts employed during these experiments produced some purine and pyrimidine nucleic acid bases in appreciable quantities. The experimental yields of the condensation studies showed that divalent metal ion present in iron oxide enhances the catalytic activity of the overall process, compared to pure iron oxide as well as some previously studied catalytic systems such as silica, alumina, manganese oxides and metal(II) octacyanomolybdate complexes. Thus, the present catalytic system may be considered as a potential prebiotic catalyst in respect of the chemical evolution and origin of life.
References
Abelson PH (1966) Chemical events on the primitive earth. Proc Natl Acad Sci 55(6):1365–1372
Agrawal S, Singh NB (2016) Removal of toxic hexavalent chromium from aqueous solution by nickel ferrite-polyaniline nanocomposite. Desalin Water Treat 57(38):17757–17766
Barks HL, Buckley R, Grieves GA, Di Mauro E, Hud NV, Orlando TM (2010) Guanine, adenine, and hypoxanthine production in UV-irradiated Formamide solutions: relaxation of the requirements for prebiotic purine nucleobase formation. ChemBioChem 11(9):1240–1243
Baudisch O (1943) The importance of trace elements in biological activity. Am Sci 31(3):211–240
Beard JS, Tracy RJ (2002) Spinels and other oxides in Mn-rich rocks from the Hutter mine, Pittsylvania County, Virginia, USA: implications for miscibility and solvus relations among jacobsite, galaxite, and magnetite. Am Mineral 87(5–6):690–698
Beletskaya I, Tyurin V (2010) Recyclable nanostructured catalytic systems in modern environmentally friendly organic synthesis. Molecules 15(7):4792–4814
Bernal JD (1951) The physical basis of life. Routledge and Paul
Bhushan B, Shanker U, Kamaluddin (2011) Adsorption of ribose nucleotides on manganese oxides with varied Mn/O ratio: implications for chemical evolution. Orig Life Evol Biosph 41(5):469–482
Bhushan B, Nayak A, Kamaluddin (2016a) Catalytic role of manganese oxides in prebiotic nucleobases synthesis from Formamide. Orig Life Evol Biosph 46(2–3):203–213
Bhushan B, Nayak A, Kamaluddin (2016b) Study of interaction and adsorption of aromatic amines by manganese oxides and their role in chemical evolution. Int J Astrobiol 16:143–155. https://doi.org/10.1017/S1473550416000203
Bindi L, Yao N, Lin C, Hollister LS, Andronicos CL, Distler VV, Eddy MP, Kostin A, Kryachko V, MacPherson GJ, Steinhardt WM, Yudovskaya M, Steinhardt WM (2015) Natural quasicrystal with decagonal symmetry. Sci Rep 5
Bockelée-Morvan D, Lis DC, Wink JE, Despois D, Crovisier J, Bachiller R et al (2000) New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron Astrophys 353:1101–1114
Borucki JG, Khare B, Cruikshank DP (2002) A new energy source for organic synthesis in Europa's surface ice. Journal of Geophysical Research: Planets 107(E11) 24–1–24-5
Botta O, Bada JL (2002) Extraterrestrial organic compounds in meteorites. Surv Geophys 23(5):411–467
Bredereck H, Gompper R, Schuh HGV, Theilig G (1959) Neuere Methoden der präparativen organischen Chemie II. 16. Synthesen mit Säure-amiden, insbesondere mit Formamid. Angew Chem 71(24):753–774
Brugger J, Meisser N (2006) Manganese-rich assemblages in the Barrhorn unit, Turtmanntal, Central Alps, Switzerland. Can Mineral 44(1):229–248
Cairns-Smith G (1992) Possible role for minerals in early organisms, Frontiers of Life, Editions Frontières. Gif sur Yvette, France, pp 119–132
Clarke, F. W., and Washington, H. S. (1924). The composition of the earth's crust (Vol. 127). US government printing office
Crovisier J (2004) The molecular complexity of comets. In: Astrobiology: Future Perspectives. Springer Netherlands, pp 179–203
Delaye L, Lazcano A (2005) Prebiological evolution and the physics of the origin of life. Phys Life Rev 2(1):47–64
Essene EJ, Peacor DR (1983) Crystal chemistry and petrology of coexisting galaxite and jacobsite and other spinel solutions and solvi. Am Mineral 68(3–4):449–455
Exon NF, Raven MD, Carlo ED (2002) Ferromanganese nodules and crusts from the Christmas Island region, Indian Ocean. Mar Georesour Geotechnol 20(4):275–297
Gallori E, Biondi E, Branciamore S (2006) Looking for the primordial genetic honeycomb. Orig Life Evol Biosph 36(5–6):493–499
Goldman A (2006) Modern ferrite technology. Springer Science & Business Media, New York
Gordon, R.; Sharov, A. (Series Volume Editors). Book entitled: Habitability of the Universe before Earth: astrobiology: exploring life on Earth and beyond. Publishers: Elsevier, Volume 1, 1st Edition (Dec 2017), Chapter 14, pages: 321–431
Gounaris Y, Litinas C, Evgenidou E (2014) A possible prebiotic function of cytosine as amino acid synthesizer. Hypothesis 12(1)
Gülaçar OF, Delaloye M (1976) Geochemistry of nickel, cobalt and copper in alpine-type ultramafic rocks. Chem Geol 17:269–280
Gupta D, Huelsekopf M, Morell Cerdà M, Ludwig R, Lippert B (2004) Complex formation of isocytosine tautomers with PdII and PtII. Inorg Chem 43(11):3386–3393
Hill-Perkins M, Jones MD, Karran P (1986) Site-specific mutagenesis in vivo by single methylated or deaminated purine bases. Mutat Res Fundam Mol Mech Mutagen 162(2):153–163
Hirao I, Kimoto M, Yamakage SI, Ishikawa M, Kikuchi J, Yokoyama S (2002) A unique unnatural base pair between a C analogue, pseudoisocytosine, and an a analogue, 6-methoxypurine, in replication. Bioorg Med Chem Lett 12(10):1391–1393
Hoover RB (2007) Microfossils of cyanobacteria in carbonaceous meteorites. In: Optical Engineering+ Applications. International Society for Optics and Photonics, pp 669408–669408
Huheey JE, Keiter EA, Keiter RL, Medhi OK (2006) Inorganic chemistry: principles of structure and reactivity. Pearson Education India
Iqubal, M. A., Sharma, R., and Kamaluddin (2015). Studies on interaction of ribonucleotides with zinc ferrite nanoparticles using spectroscopic and microscopic techniques. Karbala International Journal of Modern Science, 1(1), 49–59
Iqubal, M. A., Sharma, R., and Kamaluddin (2016). Surface interaction of ribonucleic acid constituents with spinel ferrite nanoparticles: a prebiotic chemistry experiment. RSC Adv, 6, 68574–68583
Iqubal, M. A., Sharma, R., Jheeta, S and Kamaluddin (2017). Thermal condensation of Glycine and alanine on metal ferrite surface: primitive peptide bond formation scenario. Life, 7(2), 15
Jacobs JP, Maltha A, Reintjes JG, Drimal J, Ponec V, Brongersma HH (1994) The surface of catalytically active spinels. J Catal 147(1):294–300
Jheeta S, Domaracka A, Ptasinska S, Sivaraman B, Mason NJ (2013) The irradiation of pure CH3OH and 1:1 mixture of NH3:CH3OH ices at 30 K using low energy electrons. Chem Phys Lett 556:359–364
Ji F, Zhou H, Yang Q (2008) The abiotic formation of hydrocarbons from dissolved CO2 under hydrothermal conditions with cobalt-bearing magnetite. Orig Life Evol Biosph 38(2):117–125
Karran P, Lindahl T (1980) Hypoxanthine in deoxyribonucleic acid: generation by heat-induced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry 19(26):6005–6011
Karwowski L (2012) Sołtmany meteorite. Meteorites 2:15–30
Kharisov BI, Dias HR, Kharissova OV (2014) Mini-review: ferrite nanoparticles in the catalysis. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.10.049
Kobayashi K, Ponnamperuma C (1985) Trace elements in chemical evolution, I. Orig Life Evol Biosph 16(1):41–55
Kooti M, Afshari M (2012) Magnetic cobalt ferrite nanoparticles as an efficient catalyst for oxidation of alkenes. Sci Iran 19(6):1991–1995
Kumar A, Sharma R, Kamaluddin (2014) Formamide-based synthesis of nucleobases by metal (II) Octacyanomolybdate (IV): implication in prebiotic chemistry. Astrobiology 14(9):769–779
Levy M, Miller SL, Brinton K, Bada JL (2000) Prebiotic synthesis of adenine and amino acids under Europa-like conditions. Icarus 145(2):609–613
Martins Z, Botta O, Fogel ML, Sephton MA, Glavin DP, Watson JS, Dworkin JP, Schwartz AW, Ehrenfreund P (2008) Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet Sci Lett 270(1):130–136
Mehdiye TR, Gashimov AM, Habibzade AA (2008) Electromagnetic processes in frequency-dependent resistor sheath. Fizika Cild 3:80–88
Mével C (2003) Serpentinization of abyssal peridotites at mid-ocean ridges. Compt Rendus Geosci 335(10):825–852
Millan JJ, Velilla N (1998) Mn-Fe spinel sans silicates in manganese-rich rocks from the Ossa-Morena zone, southern Iberian massif, South Western Spain. Can Mineral 36:701–711
Miller SL (1955) Production of some organic compounds under possible primitive earth conditions1. J Am Chem Soc 77(9):2351–2361
Moghaddam FM, Tavakoli G, Moafi A, Saberi V, Rezvani HR (2014) C-N bond formation using highly effective and reusable Nickel ferrite nanoparticles in water. ChemCatChem 6(12):3474–3481
Nickel EH (1973) The new mineral cuprospinel (CuFe2O4) and other spinel from oxidized ore dump at BaieVerte. Newfoundland Can Mineral 11:1003–1007
Orgel LE (2004) Prebiotic adenine revisited: eutectics and photochemistry. Orig Life Evol Biosph 34(4):361–369
Oró J (1960) Synthesis of adenine from ammonium cyanide. Biochem Biophys Res Commun 2(6):407–412
Oró J, Kamat SS (1961) Amino-acid synthesis from hydrogen cyanide under possible primitive earth conditions. Nature 190:442–443
Oró J, Kimball AP (1961) Synthesis of purines under possible primitive earth conditions. I Adenine from hydrogen cyanide. Arch Biochem Biophys 94(2):217–227
Oyelere AK, Kardon JR, Strobel SA (2002) P K a perturbation in genomic Hepatitis Delta virus ribozyme catalysis evidenced by nucleotide analogue interference mapping. Biochemistry 41(11):3667–3675
Parnell J, Baron M, Lindgren P (2006) Potential for irradiation of methane to form complex organic molecules in impact craters: implications for Mars, titan and Europa. J Geochem Explor 89(1):322–325
Ramankutty CG, Sugunan S (2001) Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Appl Catal A Gen 218(1):39–51
Reichmann HJ, Jacobsen SD (2006) Sound velocities and elastic constants of ZnAl2O4 spinel and implications for spinel-elasticity systematics. Am Mineral 91(7):1049–1054
Roy A, Debnath B, Sahoo R, Chandrakumar KR, Ray C, Jana J, Pal T (2016) Enhanced catalytic activity of ag/Rh bimetallic nanomaterial: evidence of an ensemble effect. J Phys Chem C 120(10):5457–5467
Rychagov SN, Glavatskikh SF, Sandimirova EI (1996) Ore and silicate magnetic pellets as indicators of structure and fluid regime, as well as mineral and ore formation in the present-day Baranskii hydrothermal system, Iturup Island. Geology of ore deposits 38(1):26–34
Saladino R, Crestini C, Costanzo G, Negri R, Di Mauro E (2001) A possible prebiotic synthesis of purine, adenine, cytosine, and 4 (3H)-pyrimidinone from formamide: implications for the origin of life. Bioorg Med Chem 9(5):1249–1253
Saladino R, Ciambecchini U, Crestini C, Costanzo G, Negri R, Di Mauro E (2003) One-pot TiO2-catalyzed synthesis of nucleic bases and Acyclonucleosides from Formamide: implications for the origin of life. ChemBioChem 4(6):514–521
Saladino R, Crestini C, Ciambecchini U, Ciciriello F, Costanzo G, Di Mauro E (2004) Synthesis and degradation of nucleobases and nucleic acids by formamide in the presence of montmorillonites. Chem Bio Chem 5(11):1558–1566
Saladino R, Crestini C, Neri V, Brucato JR, Colangeli L, Ciciriello F, di Mauro E, Costanzo G (2005) Synthesis and degradation of nucleic acid components by formamide and cosmic dust analogues. ChemBioChem 6(8):1368–1374
Saladino R, Crestini C, Neri V, Ciciriello F, Costanzo G, Di Mauro E (2006) Origin of informational polymers: the concurrent roles of formamide and phosphates. Chem Bio Chem 7(11):1707–1714
Saladino R, Neri V, Crestini C, Costanzo G, Graciotti M, Di Mauro E (2008) Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J Am Chem Soc 130(46):15512–15518
Saladino R, Neri V, Crestini C, Costanzo G, Graciotti M, Di Mauro E (2010) The role of the formamide/zirconia system in the synthesis of nucleobases and biogenic carboxylic acid derivatives. J Mol Evol 71(2):100–110
Saladino R, Barontini M, Cossetti C, Di Mauro E, Crestini C (2011a) The effects of borate minerals on the synthesis of nucleic acid bases, amino acids and biogenic carboxylic acids from formamide. Orig Life Evol Biosph 41(4):317–330
Saladino R, Crestini C, Cossetti C, Di Mauro E, Deamer D (2011b) Catalytic effects of Murchison material: prebiotic synthesis and degradation of RNA precursors. Orig Life Evol Biosph 41(5):437–451
Saladino R, Botta G, Pino S, Costanzo G, Di Mauro E (2012a) Genetics first or metabolism first? The formamide clue. Chem Soc Rev 41(16):5526–5565
Saladino R, Crestini C, Pino S, Costanzo G, Di Mauro E (2012b) Formamide and the origin of life. Phys Life Rev 9(1):84–104
Scappini F, Casadei F, Zamboni R, Franchi M, Gallori E, Monti S (2004) Protective effect of clay minerals on adsorbed nucleic acid against UV radiation: possible role in the origin of life. Int J Astrobiol 3(01):17–19
Schutte WA, Boogert ACA, Tielens AGGM, Whittet DCB, Gerakines PA, Chiar JE et al (1999) Weak ice absorption features at 7.24 and 7.41 MU M in the spectrum of the obscured young stellar object W 33A. Astron Astrophys 343:966–976
Shanker U, Bhushan B, Bhattacharjee G, Kamaluddin (2011) Formation of nucleobases from formamide in the presence of iron oxides: implication in chemical evolution and origin of life. Astrobiology 11(3):225–233
Sharma R, Bansal S, Singhal S (2015) Tailoring the photo-Fenton activity of spinel ferrites (MFe 2 O 4) by incorporating different cations (M= cu, Zn, Ni and co) in the structure. RSC Adv 5(8):6006–6018
Shugar D, Kierdaszuk B (1985) New light on tautomerism of purines and pyrimidines and its biological and genetic implications. J Biosci 8(3–4):657–668
Stalder M, Rozendaal A (2005) Calderite-rich garnet and franklinite-rich spinel in amphibolite-facies hydrothermal sediments, Gamsberg Zn–Pb deposit, Namaqua Province, South Africa. Can Mineral 43(2):585–599
Stoks PG, Schwartz AW (1982) Basic nitrogen-heterocyclic compounds in the Murchison meteorite. Geochim Cosmochim Acta 46(3):309–315
Timofeeva YO, Karabtsov AA, Semal VA, Burdukovskii ML, Bondarchuk N (2014) Iron–manganese nodules in Udepts: the dependence of the accumulation of trace elements on nodule size. Soil Sci Soc Am J 78(3):767–778
Toshima N, Yonezawa T (1998) Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem 22(11):1179–1201
Vallee BL, Wacker EC (1976) In: Fasman GD (ed) Handbook of Biochemistry and Molecular Biology, vol 2, 3rd edn, pp 276–292
Viswanathan B, Krishnamurthy KR, Sastri MVC (1979) Mechanism of oxidation of carbon monoxide on spinel type ferrites. J Res Inst Catalysis 27:79–88
Wächtershäuser G (1990) Evolution of the first metabolic cycles, proceedings of the National Academy of Sciences of the United States of America, vol 87, pp 200–204
Walde P (2005) Prebiotic Chemistry Edition, Topics in Current Chemistry, vol 259. Springer-Verlag, Berlin Heidelberg
Wang L, Li J, Wang Y, Zhao L, Jiang Q (2012) Adsorption capability for Congo red on nanocrystalline MFe 2 O 4 (M= Mn, Fe, co, Ni) spinel ferrites. Chem Eng J 181:72–79
Yamada H, Okamoto T (1972) A one-step synthesis of purine ring from formamide. Chem Pharm Bull 20(3):623–624
Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki KT (1978a) Reaction mechanism for purine ring formation as studied by 13 C-15 N coupling. Tetrahedron Lett 19(42):4039–4042
Yamada H, Hirobe M, Higashiyama K, Takahashi H, Suzuki KT (1978b) Detection of carbon-13-nitrogen-15 coupled units in adenine derived from doubly labeled hydrogen cyanide or formamide. J Am Chem Soc 100(14):4617–4618
Zhanpeisov NU, Leszczynski J (1999) Specific solvation effects on the structures and properties of Watson–crick and reverse Watson–crick isocytosine–cytosine and guanine–cytosine base pairs: a theoretical ab initio study. J Mol Struct THEOCHEM 487(1):107–115
Acknowledgements
This work was financially supported by the Indian Space Research Organization (ISRO), Bangalore, grant no: ISRO/RES/2/373/11-12 dated Dec. 5, 2011. I am also thankful to Mr. Joseph Paul, Director, Interfield laboratories for his kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Disclosure Statement
No competing financial interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iqubal, M.A., Sharma, R., Kamaluddin et al. Synthesis of Nucleic Acid Bases by Metal Ferrite Nanoparticles from a Single Carbon Atom Precursor Molecule: Formamide. Orig Life Evol Biosph 49, 147–162 (2019). https://doi.org/10.1007/s11084-019-09585-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-019-09585-6