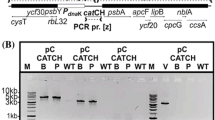
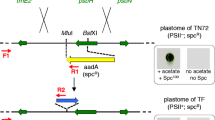
Abstract
We succeeded to create the genetically modified purple photosynthetic bacterium capable of synthesizing chlorophyll a. The results indicate that not only chlorophyll synthase, but also an enzyme for galactolipid synthesis and reaction center proteins are required for accumulating chlorophyll a.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
How oxygenic photosynthesis was established from anoxygenic photosynthesis in the course of evolution is fundamental question for elucidating evolution of early earth. One of critical factors to achieve oxygenic photosynthesis is acquisition of chlorophyll (Chl) biosynthesis, because chlorophylls can absorb much higher energy than bacteriochlorophylls do. To elucidate what components are required for early evolution of photosynthesis, we have tried to mimic evolution from anoxygenic photosynthetic bacteria to oxygenic phototrophs by direct mutagenesis. Here, our strategies for creation of purple photosynthetic bacteria capable of synthesizing chlorophyll a are summarized.
Chlorophyll and Bacteriochlorophyll Biosynthetic Pathways
Oxygenic phototrophs such as plants, algae, and cyanobacteria use Chl a, whereas most of anoxygenic phototrophic bacteria use bacteriochlorophyll (BChl) a as main pigments. Biosynthetic pathways for Chl and BChl are shared from the precursor protoporphyrin IX to the important hub intermediate chlorophyllide a. The first committed step to BChl biosynthesis in anoxygenic bacteria is catalyzed by chlorophyllide a oxidoreductase (Nomata et al. 2006; Tsukatani et al. 2013), which reduces the C7 = 8 double bond of a chlorin ring, forming a bacteriochlorin. This conversion provides more π-conjugated system, and therefore a bacteriochlorin absorb longer wavelength of light. Then, BchF and BchC catalyze the formation of the C3-acetyl group, making bacteriochlorophyllide a, and BchG (BChl synthase) esterifies a hydrophobic phytol tail at the C17 position of bacteriochlorophyllide a, resulting in BChl a (see Fig. 1). On the other hand, the committed step to Chl biosynthesis is catalyzed by ChlG (Chl synthase). ChlG esterifies a phytol tail to the C17 position of chlorophyllide a, making Chl a (Fig. 1). The chlG gene is an orthlog gene of bchG. Purple sulfur/non-sulfur bacteria produce only BChl a, and indeed have the bchG gene. This indicates that BchG does not react with chlorophyllide a but is specific to bacteriochlorophyllide a, as shown in the previous report (Kim and Lee 2010). Thus, in order to enable purple bacteria produce Chl a, we probably have to incorporate the chlG gene into them. Green sulfur bacteria synthesize both BChl a and Chl a, and they actually possess both bchG and chlG in the genome. The produced BChl a and Chl a are bound to type-I reaction centers in green sulfur bacteria, whereas purple bacteria have only type-II reaction centers. Therefore, when we try to make purple bacteria capable of producing Chl a, we should also incorporate genes for the type-I reaction center as the destination for the produced Chl a.
Importance of Galactolipids for Oxygenic Photosynthesis
It has been shown that galactolipids such as monogalactosyldiacylglycerol (MGDG) have important roles in photosynthesis. Specifically, galactolipids have established as the predominant lipid components for thylakoid membranes in cyanobacteria and chloroplasts (Dörmann and Benning 2002). Crystal structure analysis has revealed that MGDG molecules are embedded in the cyanobacterial photosystem I (PSI) reaction center (Jordan et al. 2001), indicating that they are not only bulk constituents of photosynthetic membranes, but also an integral component of type-I reaction center. Therefore, when we try to make purple bacteria synthesizing the functional type-I reaction center, we should incorporate not only genes for the type-I reaction center apo-protein but also genes for MGDG biosynthesis, because most purple bacteria do not originally synthesize MGDG.
Our Strategy for Creation of Purple Bacteria Capable of Synthesizing Chlorophyll a
In conclusion, we suggest that both type-I reaction centers and MGDG are requested constituents to produce and accumulate Chl a in purple bacteria. We, therefore, have introduced genes for type-I reaction center apo-proteins and MGDG biosynthesis as well as the chlG gene of green sulfur bacteria into purple bacteria, to mimic evolution of oxygenic photosynthesis on early earth. The results were presented at the 2nd ELSI international symposium, which was held at Tokyo on March 24–26, 2014.
References
Dörmann P, Benning C (2002) Galactolipids rule in seed plants. Trends Plant Sci 7:112–118
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411:909–917
Kim E-J, Lee JK (2010) Competitive inhibitions of the chlorophyll synthase of synechocystis sp. strain PCC 6803 by bacteriochlorophyllide a and the bacteriochlorophyll synthase of Rhodobacter sphaeroides by chlorophyllide a. J Bacteriol 192:198–207
Nomata J, Mizoguchi T, Tamiaki H, Fujita Y (2006) A second nitrogenase-like enzyme for bacteriochlorophyll biosynthesis: reconstitution of chlorophyllide a reductase with purified X-protein (BchX) and YZ-protein (BchY-BchZ) from Rhodobacter capsulatus. J Biol Chem 281:15021–15028
Tsukatani Y, Yamamoto H, Harada J, Yoshitomi T, Nomata J, Kasahara M, Mizoguchi T, Fujita Y, Tamiaki H (2013) An unexpectedly branched biosynthetic pathway for bacteriochlorophyll b capable of absorbing near-infrared light. Sci Rep 3:1217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukatani, Y., Masuda, S. Elucidation of Genetic Backgrounds Necessary for Chlorophyll a Biosynthesis Toward Artificial Creation of Oxygenic Photosynthesis. Orig Life Evol Biosph 45, 367–369 (2015). https://doi.org/10.1007/s11084-015-9446-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9446-1