Abstract
The research on the origin of life, as such, seems to have reached an impasse as a clear and universal scientific definition of life is probably impossible. On the contrary, the research on the origin of evolution may provide a clue. But it is necessary to identify the minimum requirements that allowed evolution to emerge on early Earth. The classical approach, the ‘RNA world hypothesis’ is one way, but an alternative based on nonlinear dynamics dealing with far-from-equilibrium self-organization and dissipative structures can also be proposed. The conditions on early Earth, near deep-sea hydrothermal sites, were favorable to the emergence of dissipative structures such as vesicles with bilayer membranes composed of a mixture of amphiphilic and hydrophobic molecules. Experimentally these vesicles are able to self-reproduce but not to evolve. A plausible scenario for the emergence of a positive feedback process giving them the capability of evolving on early Earth is suggested. The possibilities offered by such a process are described in regard to specific characteristics of extant biological organisms and leads for future research in the field are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extant ‘living’ organisms, after 4,000 million years of evolution (Nisbet and Sleep 2001), appear inherently different from the physical world known as ‘inanimate’. However, organisms such as viruses and prions are not easy to classify. At the beginning, when the first ‘biological’ organisms emerged, the boundaries must have been fuzzier. The term 'Life' is “too vague and general, and loaded with a number of historical, traditional, religious values”(Luisi 2006). Scientifically speaking, it is worth studying the process that led to extant biological organisms with a focus on the origin of evolution.
The key question is to identify the minimum requirements that allowed the process to emerge on early Earth when there were no cells, no peptides, not even ribonucleotides (Orgel 2000). The conventional theory is that evolution is not conceivable in the absence of a “genetic replication mechanism ensuring the maintenance, stability, and diversification of its components” as allowed by nucleic acid chemistry (Bada and Lazcano 2002; Powner et al. 2009). However, an alternative lies in evolutionary dynamics (Depew and Weber 1995). This new approach through nonlinear dynamics deals with far-from-equilibrium self-organization and dissipative structures. Even if the concept of self-organization is not easy to define (Skar 2003), it can be considered as “the spontaneous emergence of non-equilibrium structural organization on a macroscopic level, due to the collective interactions between a large number of (usually simple) microscopic subsystems” (Coveney 2003). Darwinian evolution calls for self-replicable systems with offspring that can be selected according to a natural selection process. The mechanism of evolution considered here is defined as (i) the process applies to self-organizing systems able to maintain themselves far-from-equilibrium because of favorable local conditions, (ii) the systems are able to self-reproduce, (iii) they have stabilized structural/functional properties, relatively independent of the environmental parameters, that are transmissible to their descendants (which makes them a species), and (iv) these properties may change sporadically while remaining transmissible to the descendants.
Observational and Experimental Data
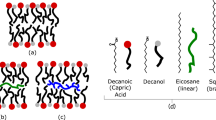
A system can be maintained far-from-equilibrium only if a flow of matter and energy is continuously supplied to it (Tabony and Job 1990) and in addition, only if the by-products can be diluted in an open milieu so that the system is not hindered by their increasing concentration. On today’s Earth there are places where such conditions are fulfilled: hydrothermal vents, such as black smoker vents and the serpentinite-hosted Lost City hydrothermal field (Kelley et al. 2005). The energy and the matter are provided by the vent fluids and the open milieu is the vast ocean. There are good arguments that many hydrothermal vents already existed on early Earth (Nisbet and Sleep 2001; Hagmann 2002), some of which were deep-sea (Kelley et al. 2005; Brasier et al. 2002). These deep-sea hydrothermal sites would have offered protective settings from the intensive solar UV radiations and the frequent massive meteorite impacts suffered by early Earth (Nisbet and Sleep 2001). The earliest fossils (- 3,235 Gyr) that were authenticated without ambiguity, i.e. as true prokaryotes, were located in a deep-sea hydrothermal site (Rasmussen 2000). For even earlier fossils (- 3,465 Gyr), there is a controversy (Brasier et al. 2002; Schopf et al. 2002). It was recently demonstrated that abiotic and morphologically complex microstructures, such as self-assembled structures, which are identical to currently accepted biogenic materials, could be synthesized inorganically (Garcia-Ruiz et al. 2003). In any case, it is plausible that the environment of early Earth was dominated by hydrothermal vents and springs spewing prebiotic organic soup into an uninhabited ocean (Brasier et al. 2002). Input of energy and matter from these deep-sea hydrothermal sites could have generated the synthesis of linear hydrocarbons and fatty acids (Holm and Charlou 2001; Rusdhi and Simoneit 2006). These compounds, which are amphiphilic or hydrophobic molecules, were probably abundant (Segré et al. 2001; Rasi et al. 2004) and their mixtures were likely to compose the membranes of relatively stable vesicles (Namani and Deamer 2008). Experimentally, such vesicles are shown to be able to self-reproduce (Rasi et al. 2004; Luisi et al. 2004; Walde 2006). However, these vesicles are not able to evolve for lack of some stabilized structural/functional membrane properties independent of the environmental parameters. Such properties would have to be transmissible to daughter vesicles after division in order to foster the emergence of vesicle species.
Proposed Scenario and Consequences
It is possible to imagine a scenario explaining how vesicles could have acquired such capabilities:
-
Amphiphilic and hydrophobic molecules were synthesized near a deep hydrothermal source in the ocean leading to the self-assembling of vesicles with bilayer membranes.
-
As the membranes were composed of a mixture of these surfactant molecules there was a huge number of theoretically possible combinations in the arrangements. Actually all combinations were not as probable: the possibility of intermolecular H-bonds (Namani and Deamer 2008) favored some.
-
Among these combinations there were specific local arrangements (Li) of the inner surface of the membrane able to enhance the reaction rate for the chemical transformation of substances Ai into Bi (Fig. 1).
-
After a long period of time, a local arrangement Ln capable of catalyzing the synthesis of Bn appeared. When Bn was formed in Ln it facilitated the formation of covalent bonds between the surfactants (Fig. 2), and a stabilized site, Sn was formed. Small molecules An and Cn entered the vesicle through the membrane while Bn could not exit the vesicle because of its chemical characteristics: size, charge, fat-solubility etc.
-
With the Sn site, the vesicle acquired the property of synthesizing Bn, relatively independently of environmental parameters (the vesicle needed to be provided with the ingredients, An and Cn). It therefore acquired a positive feedback process as Bn favored its own synthesis.
-
Where local arrangements appeared similar to Ln in other parts of the membrane, Bn stabilized them into Sn sites.
-
The transmission of the positive feedback process to daughter vesicles seems possible because the self-reproduction of vesicles, as presently understood, is described as a process in which a growing vesicle transforms its shape from a sphere into a budded shape of two spheres connected by a narrow neck, and then splits into two spherical daughter vesicles (Walde 2006). Accordingly, both Bn and Sn were likely to be present in the daughter vesicles after division, particularly if Sn sites were randomly distributed over the membrane of the parent vesicle.
Thus, a species of vesicles would have emerged, that would have survived better than the other vesicles if the stabilized sites rendered the whole membrane more stable.
The following steps may be considered as plausible for evolution after the emergence of Sn:
-
Sn catalyzed other molecules than Bn such as Bn’, a molecule with the same binding site related to Sn but a different conformation otherwise (Fig. 3). The Bn’ molecule with favorable properties for vesicle survival or reproducibility was selected.
-
New structural/functional characteristics, relatively independent of the environmental parameters, reproducible in descendants, emerged in the species.
This scenario accounts for both basic schemes of the origin of life, determinism and contingency. Determinism is represented by the formation of the vesicles with bilayer membranes composed of a mixture of amphiphilic and hydrophobic molecules, that is predictable given specific local conditions. Contingency is related to the occurrence of the positive feedback process described above, unpredictable a priori.
The possibilities for evolution created by this process are limitless. The fusion of two vesicles “where there is exchange and mixing of the water pools and formation of a new vesicle species” (Luisi 2006) may have been an efficient means of diffusion for the catalyzing capability (exchange of structural/functional memory), as bacteria did much later when exchanging their genes (exchange of genetic memory).
The catalyzing effects of the inner surface of vesicle membranes may have had another consequence. As an asymmetric mechanism it could have led to enantioselectivity. If amino acids and sugars were synthesized through this catalyzing effect, it would explain the appearance of L-amino acids and D-sugars. Thus the process could explain the impressive and well-known homochirality of natural biopolymers.
Leads for Future Research
Experimentally speaking, it is likely that, while studying the self-assembling of vesicles with a membrane composed of mixtures of amphiphilic and hydrophobic molecules, it would take thousands of years or more (or even an infinite time) to observe the emergence of a positive feed-back process. A more realistic approach would be to study a large variety of these vesicles, as proposed by authors previously cited (Namani and Deamer 2008), with the purpose of specifically investigating the potential for the inner surface of the vesicle membrane to ‘catalyze’ any kind of compound. Currently such a catalyzing effect, or at least the enhancement of the reaction rate of the chemical transformation of substance A into substance B, has been already observed in the polycondensation of amino acids and peptides initiated by activated amino acids “due to a binding of starting materials and products onto the vesicle surface” (Walde 2006) or the synthesis of RNA-like polymers from non-activated mononucleotides in lipid environments (Rajamani et al 2008). However, experiments proving the concept of a membrane catalyzing the synthesis of compounds that stabilize its local arrangements are yet to be developed. In addition it would be necessary to support the assumption that the physical conditions of high temperature and pressure that prevail near deep-sea hydrothermal sites favor the survival of vesicles with membranes composed of a mixture of surfactants and the emergence of catalyzing effects.
References
Bada JL, Lazcano A (2002) Origin of life. Some like it hot, but not the first biomolecules. Science 296:1982–1983
Brasier MD et al (2002) Questioning the evidence for Earth’s oldest fossils. Nature 416:76–81
Coveney PV (2003) Self-organization and complexity: a new age for theory, computation and experiment. Phil Trans R Soc Lond 361:1057–1079
Depew DJ, Weber BH (1995) Darwinism Evolving The MIT Press, London
Garcia-Ruiz JM, Hyde ST, Carnerup AM, Christy AG, Van Kranendonk MJ, Welham NJ (2003) Self-assembled silica-carbonate structures and detection of ancient microfossils. Science 302:1194–1197
Hagmann M (2002) Between a rock and a hard place. Science 295:2006–2007
Kelley DS et al (2005) A serpentine-hosted ecosystem: the Lost City hydrothermal field. Science 307:1428–1434
Holm NG, Charlou JL (2001) Initial indications of abiotic formation of hydrocarbons in the rainbow ultramafic hydrothermal system, mild-atlantic ridge. Earth Planet Sci Lett 191:1–8
Luisi PL, Rasi PS, Mavelli F (2004) A possible route to prebiotic vesicule reproduction. Artif Life 10:297–308
Luisi PL (2006) The emergence of life - from chemical origins to synthetic biology. Cambridge University Press, New York
Namani T, Deamer DW (2008) Stability of model membranes in extreme environments. Orig Life Evol Biosph 38:329–341
Nisbet EG, Sleep NH (2001) The habitat and nature of early life. Nature 409:1083–1091
Orgel LE (2000) Self-organizing biochemical cycles. Proc Natl Acad Sci USA 97:12503–12507
Powner MW, Gerland B, Sutherland JD (2009) Synthesis of activated pyrimidine ribonucleides in prebiotically plausible conditions. Nature 459:239–242
Rajamani S, Vlassov A, Benner S, Coombs A, Olasagasti F, Deamer D (2008) Lipid-assisted Synthesis of RNA-like polymers from mononucleotides. Orig Life Evol Biosph 38:57–74
Rasi S, Mavelli F, Luisi PL (2004) Matrix effect in oleate micelles-vesicles transformation. Orig Life Evol Biosph 34:215–224
Rasmussen B (2000) Filamentous microfossils in a 3, 235-million-year-old volcanogenic massive sulphide deposit. Nature 405:676–679
Rusdhi AI, Simoneit BRT (2006) Abiotic condensation synthesis of glyceride lipids and wax esters under simulated hydrothermal conditions. Orig Life Evol Biosph 36:93–108
Schopf JW, Kudryavtsev AB, Agresti DG, Wdowiak TJ, Czaja AD (2002) Laser-raman imagery of earth’s earliest fossils. Nature 416:73–76
Segré D, Ben-Eli D, Deamer DW, Lancet D (2001) The lipid world. Orig Life Evol Biosph 31:119–145
Skar J (2003) Introduction: self-organization as an actual theme. Phil Trans R Soc Lond 361:1049–1056
Tabony J, Job D (1990) Spatial structures in microtubular solutions requiring a sustained energy source. Nature 346:448–451
Walde P (2006) Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosph 36:109–150
Acknowledgement
I thank Robert Berman, Arnaud Lucien and Romain Tessera for helpful discussions during preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tessera, M. Life Began When Evolution Began: A Lipidic Vesicle-Based Scenario. Orig Life Evol Biosph 39, 559–564 (2009). https://doi.org/10.1007/s11084-009-9175-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-009-9175-4