Abstract
In this paper, we analyzed the structural and optical properties of quaternary semiconductor Cu2ZnSnSe4 (CZTSe) thin film. For this purpose, the structural properties of CZTSe thin film were analyzed using X-ray diffraction (XRD) and transmission electron microscopy (TEM). CZTSe nanocrystals (NCs) were formed in the kesterite phase and with good crystallinity. Optical characterization of thin film was investigated using spectroscopic measurements. Optical parameters of CZTSe film coated using the spin coating technique were determined by UV-Vis-NIR spectroscopy. The refractive index, extinction coefficient, and dielectric constant of the thin film were calculated using transmittance and reflectance data. Moreover, dispersion parameters such as oscillator energy, and dispersion energy were obtained by the Wemple DiDomenico model. For CZTSe film in the visible wavelength region, the transmission has values of 70–75%. The thin film of CZTSe has a direct band gap of 1.4 eV which is suitable for absorbed layer for solar cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is anticipated that measuring optical constants—which demonstrate the interaction of light with material—will yield more information about the spectral dependence of parameters like the refractive index and absorption coefficient, among others (Simya et al. 2022; Ahn et al. 2010). These parameters are essential for classifying materials that are appropriate for optoelectronic applications. Thin film optical properties are an incredible field of study in basic science. Material information can be revealed by optical parameters such band structure, absorbance factor, optical band gap, and index of refraction dispersion (Kumar et al. 2021). There are two fundamental factors that significantly impact the characteristics and behaviors of any semiconducting material. These are the refractive index (\(n\)) and the optical band gap (\({E}_{g}\)) (Padhy et al. 2021). Moreover, the optoelectrical and dielectric characteristics of semiconductors are significantly influenced by the refractive index, \(n\), and the extinction coefficient, \(k\) (Deokate et al. 2022). A semiconductor material with suitable optical properties is very important for the absorber layer in solar cells. Hence, it is necessary to know the optical properties of the materials to be used in solar cell construction.
Thin film technology’s increased use in solar energy conversion can be attributed to lower material consumption, its smaller size, and a wide range of photovoltaic applications [Pearce 2002; Khadir 2020; Yin et al. 2015]. Materials for thin-film solar cells frequently include cadmium telluride (CdTe) (Sinha et al. 2019) and copper indium gallium diselenide (CIGS) (Jackson et al. 2011, 2014). On the other hand, hazardous (Se, Cd) or costly (In and Ga) materials are present in CIGS and CdTe. These materials must be replaced immediately with earth-abundant, environmentally friendly substitutes with high absorption qualities (Liu et al. 2016). Therefore, I2-II-IV-VI4 quaternary chalcogenide semiconductors are reported as different from CIGS and CdTe. Cu, Zn, Sn, and S/Se are the component constituents of kesterite, a quaternary semiconductor compound. The compound has gained popularity as a thin-film absorber for solar cells due to its lower energy-generating costs in recent times.
The chalcogenide semiconductors such as Cu2ZnSnS4 (CZTS), Cu2ZnSnSe4 (CZTSe) consist of nontoxic, abundant and unexpensive materials (Katagiri et al. 2009; Dumcenco et al. 2013). Kesterite Cu2ZnSnS4 (CZTS), Cu2ZnSnSe4 (CZTSe) and Cu2ZnSn(S, Se)4 (CZTSSe) are promising materials because of suitable energy gap (1-1.5 eV) (Jiang et al. 2013; Liu et al. 2013), low cost and large absorption coefficient (~ 104cm−1) (Nakazawa 1988; Mitzi et al. 2011; Gour et al. 2017; Ko et al. 2020). Furthermore, they are p-type semiconductor and are used as an absorber layers in photovoltaic cells (Thiruvenkadam et al. 2014). In this respect, p-type CZTSe absorber material is a good choice for thin film solar cell applications.
We reported the optical properties of CZTS (Kişnişci et al. 2016), CZTSeS (Yıldırım et al. 2016), CCTSSe (Akın et al. 2021b), and CNTSSe (Yüksel et al. 2022) NCs in our previous studies. CZTSe has several benefits, including inexpensive cost, wide availability, strong photoresponse, and non-toxicity (friendly to the environment). CZTSe was therefore selected in order to examine the optical dispersion characteristics and its suitability as an absorber layer. In this paper, firstly, CZTSe nanocrystals were produced through a hot injection technique as the precursor. Secondly, Corning 1737 glass substrates were coated with nanostructured CZTSe thin films using spin coating. Transmittance and reflection measurements of nanostructured CZTSe films were taken. This work aims to explore the optical constants of the film, including the extraction coefficient constant, refractive index, absorption coefficient, and optical band gap. The optical dispersion properties of CZTSe films have not, as far as we are aware, been thoroughly reviewed. Further research is required for CZTSe film to improve the optical properties. The primary objective of the research was to formulate and ascertain these factors by the Wemple-DiDomenico model. Tetragonal CZTSe NCs were synthesized using the hot-injection method, the CZTSe NCs film on glass was prepared by spin coating, and the detailed morphological, structural, and optical properties of the films were discussed. The novelty of this research is to investigate the optical parameters and dispersion properties of compound semiconductor CZTSe NCs synthesized using the hot-injection method, which has the potential to be used for photovoltaic applications. In other words, The absorption coefficient (α) greater than 104 cm− l for VIS–NIR area and a direct band gap of 1.4 eV which is suitable for absorbed layer for solar cell of CZTSe NCs synthesized via hot injenction method route proves the novelty of our work. This work is intended to contribute to the body of knowledge regarding the optical properties of nanostructured CZTSe films.
2 Experimental details
In this work, we have examined the optical transmission and reflection spectra of a spin-coated nanocrystal CZTSe thin film. The materials used in the experiment for this purpose are as follows: Copper (II) acetate [Cu(CO2CH3)2], zinc acetate (CH3CO2)2Zn, selenium powder, isopropanol, and Ethanol [≥ 99.8%] used the synthesis of Cu2ZnSnSe4 (CZTSe) material was provided from Sigma-Aldrich. Tin (II) acetate Sn(OOCCH3)2 was obtained from Alfa Aesar. Toluene [≥ 99.7%] was obtained from VWR. Oleylamine - %80–90 (OLAM) was obtained from Across Organic.
Tetragonal CZTSe NCs were synthesized using the hot-injection method (Kus et al. 2015; Ozturk et al. 2022). In this synthesis, metal salt for the starting material and oleyl amine precursors for the capping agent were used. 1 mmol copper acetate, 0,5mmolzinc acetate, 0,5 mmol tin acetate and 20 mL OLA (80–90%) heated to 280 oC under N2 flow. To the above solution, selenium solution in oleyl amine (1 mmol Se in 2 mL OLAM) was added. After the synthesis temperature reached 280 degrees, it was continued for 30 min, and the synthesis medium was allowed to cool naturally. After the reaction medium cooled down to 120 ˚C, 2 ml of oleic acid was added to the reaction flask. Finally, the NCs were precipitated and washed with 40 mL of toluene:2-propanol (4:1) mixture to remove excess amount of surfactants. The schematic diagram of the synthesis process of CZTSe NCs is represented in Fig. 1.
XRD, SAED, and TEM analysis were performed to investigate the structural properties of the nanocrystals. The atomic percentage of elements was recorded on a Zeiss Evo model scanning electron microscope. A Bruker D8 Advance with Cu α radiation source (λ = 0.15406 Â) was used to collect XRD patterns of CZTSe NCs. A JEOL JEM-2100 transmission electron microscope was used to collect the TEM pattern and selected area electron diffraction pattern (SAED) of CZTSe NCs.
In this study, glass was used as a substrate for preparing thin film. The glass cleaning with ultrasonic cleaner is performed with ethanol, acetone, and deionized water respectively. After cleaning with ozone for 30 min, the glass substrate dried in nitrogen. Then, the CZTSe quaternary semiconductor was deposited on the glass substrate using Laurell WS-400BZ-6NPP/LITE Spin Coater with a spin speed of 1000 rpm for 1 min. The CZTSe thickness was determined 85 nm by the Veeco Dektak 150 profilometer. Using a UV-VISNIR photospectrometer (Jasco V-670 photospectrometer), the optical transmittance and reflectance spectrum of thin films have been studied over a broad spectral range, from 300 to 1000 nm.
3 Results and discussion
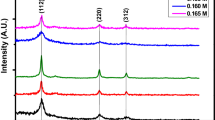
Crystal structure of CZTSe NCs was analyzed by using X-Ray Diffractometer. Figure 2 shows X-Ray diffraction (XRD) pattern and isolated crystal structure of CZTSe NCs. Characteristic peaks of CZTSe NCs are clearly seen on XRD patterns. These characteristic peaks show that the NCs are formed kesterite phase also CZTSe NCs have good crystallinity. The peaks seen in Fig. 2 correspond to (112), (204), (312), (400), (316) crystal orientations. All of the detected peaks were indexed to kesterite Cu2ZnSnSe4 (JCPDS 70-8930) based on XRD data. The red reference lines denote kesterite Cu2ZnSnSe4 (JCPDS no. 70-8930).
Energy-dispersive X-ray spectroscopy (EDS) was carried out to investigate the chemical characterization analysis of CZTSe NCs. The EDS pattern of the NCs is given in Fig. 3. As shown in Fig. 2, the elemental composition percentage of the NCs for Cu, Zn, Sn, and Se are 28%, 12%, 14%, and 46% respectively. The percentage values given here express the atomic composition and are very close to 2-1-1-4, which is the ideal composition ratio for the kesterite structure.
Figure 4a and b show TEM and HR-TEM images of the NCs. The morphological properties of the synthesized NCs were examined by TEM analysis and the results are given in Fig. 4a. Under TEM observations, it was understood that the NCs were sligthly polydisperse and their size mostly distributed between 15 and 20 nm. The small size of the nanocrystals, which is clearly seen from the TEM images, provides a good advantage in obtaining thin film morphology. Thanks to this feature, nanocrystalline thin films with uniform morphology were obtained and their properties were examined. The lattice fringes observed by HR-TEM were ~ 0.325 nm, which correspond well to the (112) plane of CZTSe. Figure 4c gives SAED pattern and display concentric rings, typical of CZTSe for planes of (112), (204) and (312). The concentric rings of CZTSe NCs correspond to XRD results. In a SAED pattern, electron beams are directed at a small, specific area of a sample. The electrons interact with the crystal lattice, resulting in diffraction patterns that provide information about the crystal structure, orientation, and phase. By analyzing the diffraction spots in the SAED pattern, researchers can deduce important structural information about the material. The rings seen in SAED analysis indicate planar diffractions. The diffractions are compatible with the diffractions obtained in the XRD analysis. The fact that both diffraction analyzes given here confirm each other increases the reliability of the material obtained as a result of the synthesis. For CZTSe nanocrystals, the SAED pattern can help determine the crystal orientation, grain size, and phase composition. Each diffraction spot corresponds to a specific set of crystallographic planes within the material. By indexing these spots and comparing them to known crystal structures, researchers can identify the crystal orientation and phase of the CZTSe nanocrystals.
After the investigation of structural properties of CZTSe, reflection and transmission spectra of CZTSe thin film were made to determine optical parameters of CZTSe thin film. Transmission (T) and reflection (R) measurements were carried out between 300 and 1000 nm wavelength and depicted in Fig. 5. According to Fig. 5, while the transmission for CZTSe film in the visible wavelength region has 70–75% values, it is 75% in the 700–1000 nm and approximately constant. Moreover, Fig. 5 depicts CZTSe NCs has a low transmissivity and reflectivity in the low wavelength. Therefore, it can be said that there is a strong absorption in this region. In a wide region range (after about 500 nm), there is no significant change for transmittance and reflectance values. Absorption values are relatively low from the visible to the near-infrared region. The material has a higher transmittance in this region. Transmission and reflection spectra of CZTSe thin films are crucial for understanding their optical properties, which are essential for various optoelectronic applications such as photovoltaics (solar cells), photodetectors, and light-emitting devices. Furthermore, transmission and reflection spectra play a pivotal role in characterizing the optical properties of CZTSe thin films, providing valuable insights into their bandgap, absorption behavior, reflection losses, and optical constants. This information is essential for optimizing the performance of photovoltaic devices and other optoelectronic applications based on CZTSe thin films.
The absorption coefficient (\(\alpha\)) was determined from the experimental data of R and T using the following equation (Kishore Kumar et al. 2009; Chan et al. 2010; Notarianni et al. 2014) :
where d parameter is the thickness of thin film. Figure 6a gives the variation of absorption coefficient (α) for CZTSe film with wavelength. As seen from the figure, \(\alpha\)is greater than 104 cm− l for VIS–NIR area. Therefore, the nanoparticle based CZTSe film is considered as a suitable material for photovoltaic solar energy conversion. The strong absorption in the low-wavelength region for CZTSe nanocrystals can be attributed to several factors such as bandgap energy, quantum confinement, surface effects, defects, dopants, size distribution, shape and morphology. CZTSe is a semiconductor material with a tunable bandgap. Nanocrystals of CZTSe can have a smaller bandgap compared to bulk CZTSe due to quantum confinement effects. This smaller bandgap leads to absorption of photons with higher energies, i.e., in the low-wavelength region. Nanocrystals have a high surface area to volume ratio. Surface states and defects can lead to additional absorption bands in the low-wavelength region. Defects, impurities, and dopants in the CZTSe nanocrystals can introduce energy levels within the bandgap, affecting the absorption spectrum. These levels can contribute to absorption at lower wavelengths. The size distribution of the nanocrystals can result in a broadening of the absorption spectrum, with some nanocrystals absorbing light at lower wavelengths. Different crystal shapes of CZTSe nanocrystals may exhibit different absorption spectra, with some shapes favoring absorption in the low-wavelength region.
For CZTSe thin films used in solar cells, having an absorption coefficient greater than 104 cm− l in the visible to near-infrared (VIS-NIR) region is significant for several reasons: A high absorption coefficient means that the CZTSe film absorbs a larger fraction of incident sunlight, allowing for more efficient utilization of the solar spectrum. This is crucial for maximizing the efficiency of solar cells since higher absorption leads to more generated electrical current. With a high absorption coefficient, CZTSe thin films can be made thinner while still absorbing a significant portion of sunlight. Thinner absorber layers reduce material usage and fabrication costs while maintaining high efficiency. In the competitive solar energy market, materials with superior absorption properties offer an advantage in terms of overall solar cell performance and cost-effectiveness. Overall, a high absorption coefficient in the VIS-NIR region is highly desirable for CZTSe thin films used in photovoltaic solar energy conversion, as it enables efficient light absorption, enhances device performance, and promotes the development of cost-effective solar cell Technologies.
To investigate the optical energy of band gap of the fabricated films, the variation of αhν with (hν–Eg)n is calculated, where \({E}_{g}\)is the optical band gap energy, hν is the photon energy, and n is either 2 for direct transition or 1/2 for indirect transition. Therefore, (αhν)2 versus hν is plotted to determine the \({E}_{g}\) of CZTSe thin film, as given Fig. 6b. As seen Fig. 6b, the linear region is obtained. \({E}_{g }\)is determined from this linear region of the plot (αhν)2 against photon energy(hν). The \({E}_{g}\) was calculated around 1.4 eV for CZTSe thin film. In addition, the energy band gaps of CZTS, CZTSSe, CCTSSe and CNTSSe materials were given in the Table 1.
CZTSe is a promising material for photovoltaic applications due to its favorable optical and electronic properties. CZTSe exhibits a high absorption coefficient for sunlight, especially in the visible and near-infrared regions of the spectrum. This characteristic makes it suitable for thin-film solar cell applications, where a thinner absorber layer can efficiently absorb sunlight, reducing material usage and cost. The bandgap of CZTSe falls within the optimal range for solar energy conversion, typically between 0.9 and 1.5 eV. This allows CZTSe to absorb a broad spectrum of sunlight, including both visible and infrared light, maximizing its efficiency in converting solar energy into electricity.
However, there are also limitations and challenges associated with CZTSe’s optical properties: While CZTSe’s bandgap falls within the optimal range for solar energy conversion, it is not as ideal as that of some other materials like crystalline silicon or gallium arsenide. This may limit its efficiency in converting sunlight into electricity compared to other materials with narrower bandgaps. CZTSe thin films often contain defects and impurities, which can adversely affect their optical properties and overall performance in photovoltaic devices. Efforts are ongoing to minimize these defects and improve the material quality. CZTSe thin films can be susceptible to degradation over time, particularly when exposed to moisture or other environmental factors. This instability can impact the long-term performance and reliability of CZTSe-based solar cells.
The refractive index (\(n\)) includes is expressed by following relation (Kishore Kumar et al. 2009).
The extinction coefficient (\(k\)), imaginary part of refractive index (\(n\)), can be determined from the α from k = αλ/4π equation. The \(n\) and \(k\) spectrums of CZTSe NCs are depicted in Fig. 7. According to Fig. 6, the \(k\) increases monotonically with increasing wavelength from 0.09 to 0,21. Then we calculated the refractive index (n) from these extinction coefficient (k) values. The \(n\) of CZTSe NCs was calculated from the values of \(k\) and R by the following equation (Kishore Kumar et al. 2009; Scragg et al. 2009; Notarianni et al. 2014; Patel et al. 2015; Zhao et al. 2017)
According to Fig. 7, the refractive index values change from 1.55 to 1.75. The spectrum of refractive index exhibits that the material doesn’t show a strong dispersion property in the measured spectral region. There is a slight increase for n values in the UV region. Moreover, the calculated values of refractive index were found to be about 1.75 in a wide spectral region. As seen from Fig. 7, the refractive index of spectrum of CZTSe is similar to reflection spectrum because of small extinction coefficient values. When the refractive index spectrum aligns with the reflection spectrum, it suggests that the material has a significant influence on the reflection behavior of light and implies that variations in the refractive index directly affect the reflection of light at the interface between the material and the surrounding medium (usually air or another transparent material). This suggests that the material has a substantial optical density, meaning it strongly interacts with light and can cause significant reflection. Furthermore, the alignment between the refractive index spectrum and the reflection spectrum indicates that the material strongly influences the reflection behavior of light and can provide valuable insights into its optical properties, index-matching conditions, presence of optical coatings, and structural characteristics. This information is essential for designing and engineering materials for various optical applications, including photovoltaics, optics, and coatings.
The complex dielectric function is one of the optical properties of any material. The complex dielectric function consists of two parts: real (\({\epsilon }_{1}\)) and imaginary (\({\epsilon }_{2}\)) part. \({\epsilon }_{1}\)and \({\epsilon }_{2}\) are dependent on extinction coefficient and refractive index of material and obtained from the following equations (Scragg et al. 2009):
The change of \({\epsilon }_{1}\) and \({\epsilon }_{2}\) versus the wavelength are shown in Fig. 8. According to Fig. 8, the \({\epsilon }_{1}\)values change between 2.4 and 3.1 and \({\epsilon }_{2}\) increases properly from 0.27 to 0.72. On the other hand, as seen from Fig. 8, the \({\epsilon }_{1}\) part is quite similar to refractive index graph. The refractive index of a material, denoted as \(n\), describes how light propagates through the material and is related to \({\epsilon }_{1}\). It is defined as the ratio of the speed of light in a vacuum to the speed of light in the material. Therefore, \({\epsilon }_{1}\) provides information about the refractive index of the material, indicating how much the material slows down the propagation of light compared to vacuum. The extinction coefficient, denoted as \(k\), describes how strongly a material absorbs light at a given wavelength. It is related to \({\epsilon }_{2}\) and is proportional to the imaginary part of the complex dielectric function. The extinction coefficient quantifies the rate at which the amplitude of an electromagnetic wave decreases as it passes through the material due to absorption.
\({\epsilon }_{1}\) indicates how light is refracted as it passes through CZTSe. A higher value of \({\epsilon }_{1}\) implies a higher refractive index, which means that light will be more strongly bent or slowed down as it enters the material. This affects phenomena such as light trapping and waveguiding in photovoltaic devices. \({\epsilon }_{2}\) reflects the absorption characteristics of CZTSe. Higher values of \({\epsilon }_{2}\) indicate stronger absorption of light, which can be advantageous for photovoltaic applications since it means more efficient conversion of light into electrical energy. However, excessive absorption can also lead to heating and energy losses, so a balance is required. Overall, \({\epsilon }_{1}\) and \({\epsilon }_{2}\) provide essential insights into how light interacts with CZTSe, which is valuable for designing and optimizing CZTSe-based optoelectronic devices such as solar cells and photodetectors.
The dispersion of the refractive index of any material is investigated using by Wemple and DiDomenico single oscillator model (DrDomenico et al. 2003; Scragg et al. 2009; Akın et al. 2021a) :
Where \({E}_{0}\) is single oscillator energy and \({E}_{d}\) is dispersion energy. To determine \({E}_{0}\)and\({E}_{d}\), 1/(n2-1) against (\(hv\))2 graph is plotted, and linear region is obtained from this graph (Fig. 9). The intercept of the linear curve at vertical axes gives where \({E}_{0}\)/\({E}_{d}\) and its slope the value of 1/\({E}_{0}{E}_{d}\). The calculated \({E}_{0}\) and \({E}_{d}\) were found as 5 and 8.16 eV, respectively. Moreover, the static refractive index,\({ n}_{0}\) can be determined by \({n_0}=\sqrt {1+\frac{{{E_d}}}{{{E_0}}}}\) as 1.62. In addition, the dispersion parameters of CZTS, CZTSSe, CCTSSe and CNTSSe materials were given in the Table 1.
4 Conclusions
In this study, we aimed to create a superior nanostructured CZTSe thin film for effective photovoltaic applications. the I2-II-IV-VI4 compound semiconductor Cu2ZnSnSe4 (CZTSe) nanocrystalline structure was obtained by hot-injection method. CZTSe nanoparticle film was coated using spin coating technique on Corning glass. Structural and optical properties of CZTSe nanocrystalline thin film have been investigated in detail. The high quality of the deposited nanostructured CZTSe thin films was confirmed by fundamental structural, morphological, and optical characterisations (e.g., X-ray diffractometer (XRD), energy dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), and UV–VIS–NIR). The X-ray diffraction peaks correspond to (112), (204), (312), (400), (316) crystal orientations. The XRD results showed that the CZTSe NCs confirm the kesterite structure with the (112) direction. Transmission and reflection measurements were used for calculation of CZTSe thin film optical constants. The absorption coefficient values of the CZTSe thin film were calculated as about 2.6–3.6 × 104 cm− 1 in visible spectrum. The direct band gap energy of the thin film was found to be 1.4 eV. This result is very compatible with the ideal band gap energy value required for the thin film absorber material in solar cell and optoelectronic material applications. The extinction coefficient (k) values were determined between 0.09 and 0.21 with increasing wavelength in the whole spectral region. The refractive index (n) values, calculated from \(k\) and \(R\)data of CZTSe thin film, vary from 1.55 to 1.75 in visible spectrum. Additionally, the dispersion of the refractive index is analyzed in terms of the Wemple DiDomenico single oscillator method. The obtained values for \({E}_{0}\), \({E}_{d}\), and \({n}_{0}\)parameters are 5, 8.16, and 1.62 eV respectively. In conclusion, we reported that the fabricated CZTSe NCs are indium free and occur earth rich elements that notably decrease the cost of cells of solar applications. Furthermore, The present report provides a new direction in fabricating nanostructured CZTSe thin films for low-cost and appropriate absorbance layer for solar cells.
Data availability
All datasets are presented in the main paper.
References
Ahn, S., Jung, S., Gwak, J., Cho, A., Shin, K., Yoon, K., Park, D., Cheong, H., Yun, J.H.: Determination of band gap energy (eg) of Cu2ZnSnSe4 thin films: On the discrepancies of reported band gap values. Appl. Phys. Lett. 97, 021905 (2010). https://doi.org/10.1063/1.3457172
Akın, Ü., Sayın, S., Tuğluoğlu, N.: Investigation of Optical and Diode parameters of 9-[(5-Nitropyridin-2-Aminoethyl) Iminiomethyl]-Anthracene Thin Film. J. Electron. Mater. 50, 2148–2156 (2021a). https://doi.org/10.1007/s11664-020-08690-x
Akın, Ü., Özel, F., Sarılmaz, A., Yüksel, Ö.F.: A new penternary semiconductor Cu2CoSn(SSe)4 nanocrystal: A study on structural, dielectric and optical properties. J. Mater. Sci. - Mater. Electron. 32, 26641–26654 (2021b). https://doi.org/10.1007/s10854-021-07041-8
Chan, C.P., Lam, H.: Preparation of Cu2ZnSnS4 films by electrodeposition using ionic liquids. Sol Energy Mater. Sol Cells. 94, 207–211 (2010). https://doi.org/10.1016/j.solmat.2009.09.003
Deokate, R.J., Chavan, H.S., Im, H., Inamdar, A.I.: Spray-deposited kesterite Cu2ZnSnS4 (CZTS): Optical, structural, and electrical investigations for solar cell applications. Ceram. Int. 48, 795–802 (2022). https://doi.org/10.1016/j.ceramint.2021.09.160
DrDomenico, M. Jr.: Oxygen-octahedra ferroelectrics. I. Theory of Electro‐optical and nonlinear optical effects. J. Appl. Phys. 40, 720–734 (2003). https://doi.org/10.1063/1.1657458
Dumcenco, D.: The vibrational properties study of kesterite Cu2ZnSnS4 single crystals by using polarization dependent Raman spectroscopy. Opt. Mater. 35, 419–425 (2013). https://doi.org/10.1016/j.optmat.2012.09.031
Gour, K.S., Singh, O.P., Yadav, A.K., Parmar, R.: Effect of NaF evaporation on morphological and structural properties of Cu2ZnSnSe4 (CZTSe) thin film deposited by sputtering from a single compound target. J. Alloys Compd. 718, 231–235 (2017). https://doi.org/10.1016/j.jallcom.2017.05.098
Jackson, P., Hariskos, D., Lotter, E., Paetel, S., Wuerz, R., Menner, R., Wischmann, W.: New world record efficiency for Cu(in,Ga)Se2 thin-film solar cells beyond 20%. Prog Photovolt. 19, 894–897 (2011). https://doi.org/10.1002/pip.1078
Jackson, P., Hariskos, D., Wuerz, R., Wischmann, W.: Compositional investigation of potassium doped Cu(in,Ga)Se2 solar cells with efficiencies up to 20.8%. Phys. Status Solidi RRL. 8, 219–222 (2014). https://doi.org/10.1002/pssr.201409040
Jiang, X., Shao, L.-X., Zhang, J., Li, D., Xie, W., Zou, C.-W.: Preparation of Cu2ZnSnS4 thin films by sulfurization of metallic precursors evaporated with a single source. Surf. Coat. Technol. 228, S408–S411 (2013). https://doi.org/10.1016/j.surfcoat.2012.05.057
Katagiri, H., Jimbo, K., Maw, W.S., Oishi, K., Yamazaki, M., Araki, H.: Development of CZTS-based thin film solar cells. Thin Solid Films. 517, 2455–2460 (2009). https://doi.org/10.1016/j.tsf.2008.11.002
Khadir, A.: Simulation of effects of defects and layers thickness on the performance of CIGS solar cells. Acta Phys. Pol. 137, 1128–1134 (2020). https://doi.org/10.12693/APhysPolA.137.1128
Kishore Kumar, Y.B., Babu, S., Uday Bhaskar, G., Raja, P.S.: Preparation and characterization of spray-deposited Cu2ZnSnS4 thin films. Sol Energy Mater. Sol Cells. 93, 1230–1237 (2009). https://doi.org/10.1016/j.solmat.2009.01.011
Kişnişci, Z., Özel, F., Yüksel, Ö.F.: Optical characterization of Cu2ZnSnS4 nanocrystals thin film. J. Mater. Sci. - Mater. Electron. 27, 10128–10135 (2016). https://doi.org/10.1007/s10854-016-5089-x
Ko, B.-S., Kim, D.-H., Hwang, D.-K., Lee, S.-J.: Effects of the annealing temperature on the properties of sulfur-graded Cu2ZnSn(S,Se)4 thin films grown by a modified two-step process. J. Ind. Eng. Chem. 82, 406–412 (2020). https://doi.org/10.1016/j.jiec.2019.11.004
Kumar, V., Singh, U.P.: Formation of CZTSSe absorber layer using thiourea treatment of CZTSe. Mater. Today. 39, 1838–1842 (2021). https://doi.org/10.1016/j.matpr.2020.06.192
Kus, M., Ozel, F., Buyukcelebi, S., Aljabour, A., Erdogan, A., Ersoz, M.: Colloidal CuZnSnSe4 – xSx nanocrystals for hybrid solar cells. Opt. Mater. 39, 103–109 (2015). https://doi.org/10.1016/j.optmat.2014.11.007
Liu, W., Guo, B., Mak, C., Li, A., Wu, X.: Facile synthesis of ultrafine Cu2ZnSnS4 nanocrystals by hydrothermal method for use in solar cells. Thin Solid Films. 535, 39–43 (2013). https://doi.org/10.1016/j.tsf.2012.11.073
Liu, X., Feng, Y., Cui, H., Liu, F., Hao, X., Conibeer, G., Mitzi, D.B., Green, M.: The current status and future prospects of kesterite solar cells: A brief review. Prog Photovolt. : Res. Appl. 24, 879–898 (2016). https://doi.org/10.1002/pip.2741
Mitzi, D.B., Gunawan, O., Todorov, T.K., Wang, K.: The path towards a high-performance solution-processed kesterite solar cell. Sol Energy Mater. Sol Cells. 95, 1421–1436 (2011). https://doi.org/10.1016/j.solmat.2010.11.028
Nakazawa K.I.: Electrical and Optical properties of Stannite-Type Quaternary Semiconductor Thin films. Jpn J. Appl. Phys. 27, 2094 (1988). https://doi.org/10.1143/JJAP.27.2094
Notarianni, M., Vernon, K., Chou, A., Aljada, M., Liu, J.: Plasmonic effect of gold nanoparticles in organic solar cells. Sol. Energy. 106, 23–37 (2014). https://doi.org/10.1016/j.solener.2013.09.026
Ozturk, T., Sarilmaz, A., Akin, S., Dursun, H., Ozel, F.: Quinary Nanocrystal-based passivation strategy for high efficiency and stable Perovskite Photovoltaics. Solar RRL. 6, 2100737 (2022). https://doi.org/10.1002/solr.202100737
Padhy, S., Mannu, R., Singh, U.P.: Graded bandgap structure of kesterite material using bilayer of CZTS and CZTSe for enhanced performance: A numerical approach. Sol Energy. 216, 601–609 (2021). https://doi.org/10.1016/j.solener.2021 01.057
Patel, K., Shah, D.V.: Influence of deposition parameters and annealing on Cu2ZnSnS4 thin films grown by SILAR. J. Alloys Compd. 622, 942–947 (2015). https://doi.org/10.1016/j.jallcom.2014.11.019
Pearce, J.M.: Photovoltaic-A path to Sustainable futures. Futures. 34, 663–674 (2002). https://doi.org/10.1016/S0016-3287(02)00008-3
Scragg, J.J., Dale, P.J.: Synthesis and characterization of Cu2ZnSnS4 absorber layers by an electrodeposition-annealing route. Thin Solid Films. 517, 2481–2484 (2009). https://doi.org/10.1016/j.tsf.2008.11.022
Simya, O.K., Vijayaraghavan, T., Subramanian, B., Ashok, A.M.: Efect of RF sputter power on deposition of CZTX (X = S, Se) active layer without selenization/sulfurization, for solar cell applications. J. Alloys Compound. 898, 162838 (2022). https://doi.org/10.1016/j.jallcom.2021.162838
Sinha, T., Lilhare, D., Khare, A.A.: Review on the improvement in performance of CdTe/CdS thin-film solar cells through optimization of structural parameters. J. Mater. Sci. 54, 12189–12205 (2019). https://doi.org/10.1007/s10853-019-03651-0
Thiruvenkadam, S., Jovina, D.L., Rajesh, A.: The influence of deposition temperature in the photovoltaic properties of spray deposited CZTS thin films. Sol. Energy. 106, 166–170 (2014). https://doi.org/10.1016/j.solener.2014.02.041
Yin, L., Cheng, G., Feng, Y., Li, Z., Yang, C., Xiao, X.: Limitation factors for the performance of kesterite Cu2ZnSnS4 thin film solar cells studied by defect characterization. RSC Adv. 5, 40369–40374 (2015). https://doi.org/10.1039/C5RA00069F
Yıldırım, M., Özel, F., Tuğluoğlu, N., Yüksel, Ö.F.: Optical characterization of Cu2ZnSnSe4-xSx nanocrystals thin film. J. Alloys Compd. 666, 144–152 (2016). https://doi.org/10.1016/j.jallcom.2016.01.107
Yüksel, Ö.F., Özel, F., Akın, Ü.T.: Structural, optical and dispersion studies on Cu2NiSn(SSe)4 nanocrystals thin films. Phys. B. 625, 413530 (2022). https://doi.org/10.1016/j.physb.2021.413530
Zhao, J., Liang, G., Zeng, Y., Fan, P., Hu, J., Luo, J.: The influence of sequence of precursor films on CZTSe thin films prepared by ion-beam sputtering deposition*. J. Semicond. 38, 023002 (2017). https://doi.org/10.1088/1674-4926/38/2/023002
Acknowledgements
Z. Kişnişci acknowledges Selçuk University BAP Project Office, Konya, Turkey for a financial support (FEN-BAP 14101020).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by the Selçuk University BAP Project Office (No: FEN-BAP 14101020).
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and structure analysis were performed by FÖ and ZK. Optical analysis was done by ZK, NT and ÖFY. The manuscript was written by ZK. The experimental tests were carried out by ZK, ÖFY, NT and FÖ. The manuscript was revised by ZK, ÖFY, NT and FÖ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Authors declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kişnişci, Z., Özel, F., Tuğluoğlu, N. et al. Structural and optical properties of Cu2ZnSnSe4 nanocrystals thin film. Opt Quant Electron 56, 1239 (2024). https://doi.org/10.1007/s11082-024-07136-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-07136-6