Abstract
Highly sensitive biosensor based on D-shaped photonic crystal fiber (PCF) with plasmonic grating is introduced and analyzed. The suggested structure is tested using four different grating structures (rectangular, triangular, circular, or elliptical) on the polished surface of the D-shaped PCF. The sensing operation depends on surface plasmon resonance mechanism where the analyte refractive index (RI) is utilized to control the coupling between the core mode and surface plasmon mode via phase matching phenomenon. Rhodium is employed as a plasmonic material to induce the SPMs. The resonance (i.e., phase matching) wavelength is a function of the analyte RI. The geometrical parameters of the proposed structure are optimized using full vectorial finite element method to enhance the sensor sensitivity. The proposed biosensor can be utilized in the detection of different cancerous Basel, Breast and Cervical cells. The performance of the reported biosensor is investigated in terms of sensitivity, linear response, and fabrication tolerance. The reported biosensor has high sensitivities of 19,750 nm/RIU, 20,428 nm/RIU and 20,041 nm/RIU for the detection of Basel, Breast and Cervical cancer cells, respectively. The presented biosensor is a good candidate for biological sample detection and organic chemical sensing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the world health organization (WHO), cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths (https://www.who.int/health-topics/cancer#tab=tab_1) ([CSL STYLE ERROR: reference with no printed form.]). The most common cancer types are Breast, lung, colon rectum and prostate cancers. However, many types of cancer can be cured if detected early and treated effectively (https://www.who.int/health-topics/cancer#tab=tab_1). For that reason, the research on early cancer detection has gained tremendous interest to the academic researchers. Photonic Crystal Fibers (PCFs) have demonstrated a great interest due their unique optical properties that can overcome some of the fundamental limitations of standard optical fibers. Usually, PCF is made of purely silica background material with periodic arrangement of air holes in the cladding region running along the fiber (Zhang et al. 2018). The PCF, as a good transmission medium, guides the light by two different guiding mechanisms; index-guiding and photonic bandgap (PBG) guiding. In the index guiding PCFs, light is guided in a high index core region that is surrounded by a low index cladding region. However, in PBG based PCFs, light is confined in a low-index core by the reflection from a periodic cladding with low transmission loss for a finite spectral window (Zhang et al. 2018). Because of their novel optical guiding mechanism and diverse design, PCFs can be utilized in different promising fields and applications (Cai et al. 2016; Li and Luo 2016; Hameed and Obayya 2011). Index-guiding PCFs have interesting properties that include endlessly single mode guidance (Birks et al. 1997), large mode area (Knight et al. 1998), large numerical aperture (Wadsworth et al. 2003), and high birefringence (Hansen et al. 2001; Ju et al. 2003; Kim et al. 2012). Therefore, PCFs have a pivotal role in polarization handling devices (Hameed et al. 2010, 2013), optical communications and communication networks (Chaudhary and Amphawan 2018). In addition, PCFs can be used as sensing media for gas, humidity, voltage, current, magnetic field, and power (Filippov et al. 2000; Li et al. 2013a; Morshed et al. 2015; Rabee et al. 2019; El-Saeed et al. 2019)). Further, PCFs are employed in different kinds of disease detection through salinity sensing (Vigneswaran et al. 2018), pH (Li et al. 2013b), DNA (Sun et al. 2014), cholesterol (Soylemez et al. 2015) and glucose sensing (An et al. 2017). PCFs have been also used for oncovirus (a virus that can cause cancer) detection (Mishra et al. 2020). Surface plasmon resonance (SPR) and localized SPR are the two main approaches employed in plasmonic sensing field. SPR based sensors are widely employed for their simplicity and excellent RI sensitivity compared to localized SPR sensors. Plasmonic biosensors are powerful and effective tools that have been widely used in biosensing field (Al Mahfuz et al. 2020; Paul et al. 2020). In this context, Sun et al. demonstrated that Breast cancer cells were sensitively detected by fluorescence biosensors and square-wave voltammetry after magnetic accumulation and fluorescent labeling (Sun et al. 2021). Additionally, Niciński et al. (2019) have used the silver nanoparticles coated with an ultrathin shell of silica to improve the detection sensitivity of analyzed tumor cells via the shell-isolated nanoparticle-enhanced Raman spectroscopy. A novel Basal cell carcinoma detection has been performed via high-definition optical coherence tomography (Li et al. 2016). Further, the detection of cancer biomarkers using an electrochemical biosensor has been reported efficiently with lower cost compared to mammography, Breast ultrasound, and Breast MRI examination (Hong et al. 2022). Among all existing biosensors, optical biosensors attract greater attention due to their real-time operation, ultra-high sensitivity, wide dynamic detection range, negligible electromagnetic interference. In addition, optical biosensors can be designed without the need of a reference electrode with no electrical hazards. Further, they are highly stable, have multiplexing capabilities, and relatively cheap (Usman et al. 2021). Plasmonic optical fiber-based immunosensor for lung tumor detection has been proposed in Ribaut et al. (2017). While the Bragg grating acts as a mirror for the light propagating through the core mode, a long-period fiber gratings (LPFG) couples the light from fundamental guided modes to the other forward co-propagating cladding modes. Quero et al. (2011) experimentally showed LPFGs can be used for the chemical sensing applications, depending on the nature of the material deposited onto the flat surface of the etched D-fiber. Peng et al. (2015) numerically demonstrated a D-shaped microstructured optical fiber biosensor with rectangular lattice with two large air holes. It has been shown that the sensing performance is affected by two large leaky channels and birefringence between the two polarized nodes (Peng et al. 2015). Further, D-shaped PCF-SPR biosensor has been proposed based on graphene layer between the silver layer and D-shaped PCF (Tong et al. 2018). Recently, the dimensions of D-shaped bare fiber probes has been optimized for RI sensing by varying the probe length of a multi-mode fiber for the biomolecular sensing (Virk et al. 2022). A long period fiber grating sensor LPFG is a periodic variation of RI in the fiber core when the light can be coupled the fundamental core mode to the cladding modes. Therefore, a set of resonant attenuation bands are supported at discrete wavelengths in the LPFG transmission spectrum (Shu et al. 2002). A LPFG with maximum enhancement of evanescent field has been designed by working near the turn-around point (TAP) of lowest order symmetric cladding mode. The LPFG was fabricated using a point-by-point technique. The diameter of the LPFG was reduced by chemical etching, for the maximum enhancement of the evanescent field (Dey et al. 2020). A new method to enhance the sensitivity of LPFG at the TAP has been presented (Dey et al. 2020). The LPFG sensor has been etched until the sidelobes of dispersed LP0,2 cladding mode appeared near TAP. The LPFG sensitivity was found to be 16,044 nm/SRIU (surrounding refractive index units) in the RI range from 1.333 to 1.3335 (Dey et al. 2022). A gold/TiO2 coated photonic crystal fiber (PCF) biosensor for sensing breast cancer cells is studied (Verma et al. 2022). The impact of the presence of thin metallic film, air holes pitch and RI of the analytes are optimized through supervised machine learning approach with low mean square error to use as blood related disease detection device. A SPR based (PCF) biosensor is designed for early cancer detection (Yasli 2021). The spectral interrogation and amplitude methods are used to detect the refractive index (RI) variations of cancer cells. The highest sensitivities are 7142. 86 nm/RIU for 1.392 to 1.401 RI range. Additionally, Ayyanar et al. have proposed an optical biosensor based on dual core PCF for the detection of cancer cells in Cervical, Breast and Basel parts (Ayyanar et al. 2018). Further, Jabin et al. (2019) have reported a bowl-shaped plasmonic PCF mono-core sensor for cancer detection.
Rhodium has higher plasma frequency than Gold and Silver (Adachi 2012). Thus, it is not commonly used in the NIR wavelength regime. Instead, it has been used as a reflective or protective coating in optical applications and as a catalyst in various chemical reactions in UV wavelength range. However, in recent years, Rh has been employed as an active SPR material in the NIR range in many photonics applications. In this context, a bi-metallic grating of Rh and Ag is used to design an ultra-compact transverse electric (TE) pass polarizer (Hiza et al. 2023). Additionally, an I-shaped Rh nanomaterial has been investigated in Yaru et al. (2023) via the finite-difference time-domain method. It has been found that multiple plasmonic resonances can be excited in different wavelength regions (Yaru et al. 2023). The generation of high-intensity electric fields between nanorods provides a potential platform for nanoantenna and metal-enhanced fluorescence applications (Yaru et al. 2023). In addition, the stability of Rh, as well as its interesting plasmonic properties, make it attractive for use as substrate for surface-enhanced Raman scattering (SERS) (Zettsu et al. 2006). Among the platinum group metals, Rh is known as a valuable element for automotive catalysts due to its powerful catalytic function and it is vital for the catalytic reaction to purify exhaust gas from gasoline engines (Goto et al. 2014). A bi-metallic layer of Rh/Ag based Magnesium Fluoride (MgF2) prism-coupled has been proposed as gas sensor (Mishra and Mishra 2016). Thus, Rh has proved to be a viable candidate for plasmonic based sensing applications.
Here in, a novel D-shaped PCF with Rhodium grating (RG) is utilized for cancer detection purposes. In this study, Basel, Breast, and Cervical cells are studied using D-shaped PCF structure. The sensing operation is studied by detecting the RI variation of each cell to provide the highest sensitivity and prolong in early determination cancer biomarkers. In the proposed D-shaped PCF, the mode coupling is tested for both normal and cancerous cells by using full vectorial finite element method (FVFEM) (Obayya et al. 2003) via COMSOL Multiphysics software package (https://www.comsol.com/). Additionally, four grating structures are tested to achieve the maximum sensitivity. The studied grating structures have circular, triangular, elliptical, and rectangular shapes. It is worth mentioning that the rectangular Rhodium grating (RRG) structure gives the maximum sensitivities of 16,350 nm/RIU, 16,642 nm/RIU, and 16,500 nm/RIU for Basel, Breast, and Cervical cancerous cells, respectively. The sensitivity performance of the proposed biosensor is analyzed and found better in comparison to the previously published articles in Ayyanar et al. (2018), Jabin et al. (2019), Mishra et al. (2020), Vijayalakshmi and Manimegalai (2021), and Yasli (2021). Therefore, the reported biosensor is a good candidate in early cancer detection.
2 Materials and methods
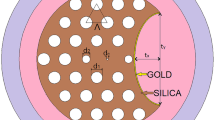
The schematic cross-sectional view of the proposed SPR-PCF biosensor is shown in Fig. 1. The reported PCF has two hexagonally arranged rings of air holes in a silica background material. Air holes with two different diameters are used where the inner small air holes have a radius of \({{\text{r}}}_{1}\) while the outer large air holes have a radius of \({{\text{r}}}_{2}\). The values of \({{\text{r}}}_{1}\) and \({{\text{r}}}_{2}\) are chosen to control the light confinement through the PCF design (Momota and Hasan 2018). The distance between the adjacent air holes (lattice constant) is \(\Lambda =2.3\,\upmu\mathrm{m}\). The etched surface is located at a distance \({{\text{L}}}_{{\text{y}}}=3.25\,\upmu\mathrm{m}\) from the center of the PCF as may be seen in Fig. 1. The D-shaped PCF has a channel above the polished surface of thickness \({\text{d}}=3\,\upmu\mathrm{m}\) to house the analyte as depicted in Fig. 1. Chemically inactive and stable Rhodium (Rh) is employed as a plasmonic material in this design. Rhodium is a soft silvery durable transition metal with a face-centered cubic crystal structure at standard conditions of temperature and pressure (Abarca-Quesada et al. 2021; Meyers and Myers 1997). Further, Rh is utilized as an alloying agent for hardening and improving the corrosion resistance, in electroplating (Czerczak et al. 2012), and in other applications (Ahmadivand et al. 2016; Gutiérrez et al. 2018; Zhang et al. 2016) based on its plasmonic properties.
The selected Rhodium grating (RG) is placed over the planar surface of the D-shaped PCF to enhance the sensing performance. The grating constant is \({\text{s}}=500\,\mathrm{nm}\), with a rectangular height \(\mathrm{h\,of\,}100\,\mathrm{nm}\), a base length \(\mathrm{L\,of\,}500\,\mathrm{nm}\) and the number of grating elements is \({{\text{G}}}_{{\text{n}}}=5\) as depicted in Fig. 1a. In the proposed biosensor, we have tested four RG structures; triangular, circular, elliptical, and rectangular as shown in Fig. 1a. The ultimate goal is to achieve a simple and fabrication-feasible biosensor with high sensing performance. It is worth mentioning that the Sellmeier equation of fused silica (Akowuah et al. 2012) depicted in Eq. (1) is utilized in the current work.
where n is the refractive index of fused silica and \(\uplambda\) is the wavelength in microns. The Sellmeier coefficients \({{\text{A}}}_{1}\), \({{\text{A}}}_{2}\), \({{\text{A}}}_{3}\), \({{\text{B}}}_{1}\), \({{\text{B}}}_{2}\), and \({{\text{B}}}_{3}\) are reported in (Akowuah et al. 2012). The dispersion characteristics of the Rh in terms of refractive index “n” and extinction coefficient “ĸ” have been measured as functions of photon energy “E” in Adachi (2012), Weaver et al. (1977). In our work, the complex refractive index of Rh is taken from Weaver et al. (1977), in which the measured data covers the utilized wavelength range in this work. The large extinction coefficient of Rh proves that it is a heavily lossy material and can’t be used in bulk (only a thickness of 100 nm is employed in our device). However, the larger negative real part of its permittivity ensures the higher sensitivity of the device.
It is important to mention that the FVFEM via COMSOL Multiphysics software package (https://www.comsol.com/) is employed to evaluate the characteristics of the quasi-TM core guided mode and SP modes supported by the proposed D-PCF structure. The full vector finite element method (FVFEM) is a powerful tool for accurate investigation of optoelectronic devices (Koshiba and Tsuji 2000). The FEM uses a variational expression formulated from Maxwell’s equations where the problem is reduced to a standard eigenvalue matrix equation. Iterative techniques can be used to solve this matrix to calculate the field profiles and propagation constants of the supported modes. Through the FEM solution, the computational domain is discretized into smaller sub-domains (elements). Further, the FEM has the ability to handle geometrically complex domains by subdividing them into smaller and nonuniform subdomains, called ‘finite element’. The solutions are formulated at generated element nodes to obtain the solution for the original whole domain. The FEM can be used to solve the wave equation in scalar, semi-vectorial, or full-vectorial manner. For the full vector finite element solution, the wave equation takes the form (Kawano and Kitoh 2004):
where \(\widehat{{\text{p}}}\) and \(\widehat{{\text{q}}}\) represent \({\widehat{\upmu }}^{-1}\) and \(\widehat{\upvarepsilon }\) when \(\overline{\Phi }\) denotes the electric field \({\overline{\text{E}}}\) and \({\widehat{\upvarepsilon }}^{-1}\) and \(\widehat{\upmu }\) when \(\overline{\Phi }\) denotes the magnetic field \({\overline{\text{H}}}\); \({{\text{k}}}_{0}\) is the free space wavenumber (Kawano and Kitoh 2004). The domain of the differential equation is discretized, and the overall solution is obtained by minimizing the error residual of the differential equation (Kawano and Kitoh 2004) Discretizing the cross section of the waveguide using the proper element (interpolation functions) and applying one of the previously mentioned FEM procedures, one can obtain
where \(\left[K\right]\) and \(\left[M\right]\) are the global stiffness and mass matrices can be formed based on the type of interpolation function used, \(\left\{ \Phi \right\}\) is the global electric or magnetic field vector and \(\left\{ 0 \right\}\) is the null vector. In the proposed work, the whole computational domain is \(12\,\upmu\mathrm{m}\times 12\,\upmu\mathrm{m}\), where the number of triangular mesh elements, degrees of freedom and minimum element size are set to 109,154 elements, 765,109, and \(3.72\,\mathrm{nm}\), respectively to ensure the high accuracy of the numerical results.
It is well-known that cancer may result from abnormal proliferation of different kinds of cells in the human body leading to more than a hundred distinct types. In this work, different types of cancer cells are studied including Basel, Breast, and Cervical parts. The RIs of various cancerous cells vary from 1.38 to 1.4 while those of normal cells change from 1.36 to 1.39. All RIs of different Basel, Breast and Cervical cells and those of normal cells are depicted in Table 1. The simulation is performed for analyte RI range from 1.36 to 1.399 to cover all the studied cell types while the operating wavelength range extends from 1000 to 3000 nm. The effect of different geometrical parameters on the performance of the proposed biosensor is carried out in the upcoming sections to maximize the sensor sensitivity.
The fabrication process of the reported D-shaped PCF can be performed using the conventional drawing technique (Azab et al. 2022; Chen et al. 2004). Then, the upper part of the PCF is polished, and the PCF can be then spliced between two single-mode fibers (SMFs) (Amouzad Mahdiraji et al. 2014). It is worth noting that computer-controlled polisher can accurately define the polishing length and depth. It is feasible to create tiny grating constants in hundreds of nanometers using X-ray photolithography (XRL) (Cerrina 2000), electron beam lithography (EBL) (Kolodziej and Maynard 2012), or a focused ion beam (FIB) (André et al. 2014). The latter is a commercially available technology that has been applied to optical fibers, leading to new and interesting ways of creating very small and short optical fiber devices (André et al. 2014). Therefore, the plasmonic gratings can be deposited on the D-shaped surface via FIB technology (Amouzad Mahdiraji et al. 2014; Yan et al. 2013). To recapitulate, a novel SPR-PCF biosensor with high sensitivity and a fabrication-friendly model that can be contrived through the Stack-and-Draw method. The perfectly matched layer (PML) encompassing the fiber ensures the absorbance of the scattered evanescent field (Dey et al. 2020). Therefore, the authors believe that the suggested D-shaped PCF with grating structure can be achieved experimentally using current.
3 Results and discussion
3.1 Performance analysis
The present research aims to achieve early detection of cancer cells via an SPR-PCF biosensor by exploiting the interaction between the evanescent field of the core mode with one of the SPMs generated at the plasmonic grating constructed on the RG surfaces. In this study, three types of cell groups are considered as analytes whose RIs are summarized in Table 1. The wavelength sensitivity is defined as the shift of the resonance wavelength \((\Delta {\uplambda }_{{\text{p}}})\) divided by the corresponding analyte RI variation \((\Delta\mathrm{n})\) (Nayak 2023) is given by:
It is worth mentioning that the simulations are carried out to study the sensitivity variation by employing four different grating structures with triangular, circular, elliptical, and rectangular shapes. The geometrical parameters are initially taken as \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\),\({\text{h}}=0.1\,\upmu\mathrm{m}\),\({\text{L}}=0.5\,\upmu\mathrm{m}\),\({\text{s}}=0.5\,\upmu\mathrm{m}, {{\text{r}}}_{1}=0.4\,\upmu\mathrm{m}\),\({{\text{r}}}_{2}=0.8\,\upmu\mathrm{m}\), and \({{\text{G}}}_{{\text{n}}}=5\) for all considered structures (triangular, circular, elliptical, and rectangular). The initial structural parameters are chosen to keep the fabrication feasibility of the suggested SPR-PCF biosensor.
First, the supported modes by the suggested structure are studied and analyzed in detail. In this investigation, the fundamental quasi-TM mode supported by the PCF-core and the SPMs constructed at the metal/dielectric interfaces are introduced. The selected modes are analyzed in terms of the real part of the effective index and the confinement loss. Figures 2, 3, and 4 depict the confinement losses of the quasi-TM core mode for normal and cancerous Basel, Breast, and Cervical cells, respectively. The wavelength dependent confinement loss can be calculated from Eq. (3) (Alrayk et al. 2021):
where \({\text{Im}}\left({{\text{n}}}_{{\text{eff}}}\right)\) is the imaginary part of the complex effective index of the corresponding mode.
Wavelength dependent effective indices (\({{\text{n}}}_{{\text{eff}}}\)) and loss spectra for the quasi-TM mode supported by the PCF structure in both normal and cancerous Basel cells for different studied grating structures; a triangular, b circular, c elliptical and d rectangular. The geometrical parameters are \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\),\({\text{h}}=0.1\,\upmu\mathrm{m}\),\({\text{L}}=0.5\,\upmu\mathrm{m}\),\({\text{s}}=0.5\,\upmu\mathrm{m}, {{\text{r}}}_{1}=0.4\,\upmu\mathrm{m}\),\({{\text{r}}}_{2}=0.8\,\upmu\mathrm{m}\), and \({{\text{G}}}_{{\text{n}}}=5\) for all considered structures
Wavelength dependent effective indices (\({{\text{n}}}_{{\text{eff}}}\)) and loss spectra for the quasi-TM mode supported by the PCF structure using both normal and cancerous Breast cell for different studied grating structures; a triangular, b circular, c elliptical and d rectangular. The geometrical parameters are \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\),\({\text{h}}=0.1\,\upmu\mathrm{m}\),\({\text{L}}=0.5\,\upmu\mathrm{m}\),\({\text{s}}=0.5\,\upmu\mathrm{m}, {{\text{r}}}_{1}=0.4\,\upmu\mathrm{m}\),\({{\text{r}}}_{2}=0.8\,\upmu\mathrm{m}\), and \({{\text{G}}}_{{\text{n}}}=5\) for all considered structures
Wavelength dependent effective indices (\({{\text{n}}}_{{\text{eff}}}\)) and loss spectra of the quasi-TM mode supported by the PCF structure using both normal and cancerous Cervical cell for different studied grating structures; a triangular, b circular, c elliptical and d rectangular. The geometrical parameters are \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\),\({\text{h}}=0.1\,\upmu\mathrm{m}\),\({\text{L}}=0.5\,\upmu\mathrm{m}\),\({\text{s}}=0.5\,\upmu\mathrm{m}, {{\text{r}}}_{1}=0.4\,\upmu\mathrm{m}\),\({{\text{r}}}_{2}=0.8\,\upmu\mathrm{m}\), and \({{\text{G}}}_{{\text{n}}}=5\) for all considered structures
Table 2 summarizes the resonance wavelengths that correspond to phase matching points between the quasi-TM core mode and the SPM for normal and cancerous cells according to the results shown in Figs. 2, 3, and 4. Generally, the resonance wavelength in each studied cell type/structure is shifted as the RI is changed from the normal cell to the cancerous cell as depicted in Table 2. Therefore, the suggested design can be used for detecting the variation in RI of each cell sample. The insets in Fig. 2 show the field profiles for both quasi-TM core mode and SPMs that correspond to the four studied structures.
It is worth noting that these field profiles are plotted at the phase matching wavelength according to each studied cell type/structure. At the phase matching wavelengths, the \({{\text{n}}}_{{\text{eff}}}\) of the quasi-TM core mode and that of the SPM are nearly equal as shown in Figs. 2, 3, and 4. The intersections of the \({{\text{n}}}_{{\text{eff}}}\) of the TM and SPM core modes are shown in Fig. 2. Also, it may be seen from Figs. 2, 3, and 4 that the effective index decreases gradually with the increase in wavelength for both normal and cancerous cells. The maximum loss of the supported modes using normal and cancerous cells are equal in the different studied RG structures.
The corresponding sensitivities obtained for different studied cell types using different RG structures are also summarized in Table 2. From the obtained results depicted in Table 2, it may be realized that a higher sensitivity in all considered cell types is obtained by using the rectangular RG (RRG) structure. The calculated sensitivities (using Eq. (2)) of the suggested SPR-PCF biosensor with RRG are 16,350 nm/RIU, 16,642 nm/RIU, and 16,500 nm/RIU for Basel, Breast, and Cervical cell, respectively. From the obtained results, it may be seen that the SPR-PCF biosensor with RRG can be employed as a good candidate for accurate sensing of cancer cells. In the following section, the different geometrical parameters are studied in detail to optimize the biosensor operation in terms of sensitivity (S). It is worth noting that among the four studied grating structures, RRG structure induces the highest sharpness of the loss peak. Therefore, the RRG structure will be only considered in the upcoming simulations.
In order to show the superiority of Rh on Au, a study is performed using Au instead of Rh on the proposed rectangular grating D-shaped PCF structure. It is worth noting that this study is done using the optimum geometrical parameters in the case of Breast cell. The confinement loss spectra of the quasi-TM core mode using the normal and cancerous cells of the Breast cell are determined. The calculated sensitivity by using Au (instead of Rh) is 7,928.6 nm/RIU which is much smaller than that of using Rh (20,428 nm/RIU). Thus, employing Rh makes the sensitivity ~ 2.5 times that of using Au.
3.2 The effect of structural parameters
To maximize the sensitivity, optimization of the proposed structure using rectangular grating is essential. The grating thickness (h) as shown in Fig. 1 is expected to have a large effect on the mode characteristics and can control the sensing operation. In this study, other geometrical parameters are taken as \({\text{L}}=450\,\mathrm{nm}\),\({\text{s}}=500\,\mathrm{nm}, {{\text{r}}}_{1}=400\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\). Figure 5 depicts the variation of the confinement losses of the quasi-TM core mode with λ for the normal cell and different cancerous Basel, Breast and Cervical cells for different grating thicknesses. It may be seen from Fig. 5 that the resonance wavelength is shifted toward longer wavelengths with the increase of h. It is worth noting that increasing h would increase the overall metal surface seen by the analyte. Accordingly, the interaction between the SPM and the analyte is enhanced and the optical properties especially neff of the SPM is greatly modified. This in turn would increase the sensitivity of the proposed biosensor. The calculated sensitivities of the Basel, Breast, and Cervical cancer cells are 17,600 nm/RIU, 17,928 nm/RIU, and 17,708 nm/RIU, respectively at \({\text{h}}=150\,\mathrm{nm}\). By comparing these values with those depicted in Table 2 at \({\text{h}}=100\,\mathrm{nm}\), it is found that the highest sensitivity is obtained at \({\text{h}}=150\,\mathrm{nm}\). The results obtained in Fig. 5 reveal that the sensitivity increases by increasing h for all selected cancerous cells. Thus, h is fixed at \(150\,\mathrm{nm}\) to keep the sensitivity as high as possible and keep the fabrication feasibility.
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different rectangular grating height h using the normal and cancerous cells of the Basal, Breast and Cervical cells. The geometrical parameters are fixed to \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\),\({\text{L}}=450\,\mathrm{nm}\),\({\text{s}}=500\,\mathrm{nm}, {{\text{r}}}_{1}=400\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\)
Figure 6 shows the wavelength-dependent confinement losses of the quasi-TM core mode at different RG length (L) in both, normal and cancerous cells. It is worth mentioning that three different values of L (\(450\,\mathrm{nm}\), 500 nm and \(550\,\mathrm{nm}\)) are studied to show its effect on the loss behavior of the quasi-TM mode. In this study, the geometrical parameters are taken as \({\text{h}}=150\,\mathrm{nm}\),\({\text{s}}=500\,\mathrm{nm}, {{\text{r}}}_{1}=400\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\). It is expected that reducing L would enhance the sensitivity as small L gives a huge area for interaction between the SPM and the analyte. On the other hand, increasing L would reduce the interaction region between them. In this study, the highest sensitivities are obtained at \({\text{L}}=450\,\mathrm{nm}\) and found to be 18,000 nm/RIU, 18,500 nm/RIU, and 18,208 nm/RIU for Basel, Breast, and Cervical cell, respectively. Thus, L is fixed at \(450\,\mathrm{nm}\) to keep the sensitivity at its highest levels.
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different rectangular grating length L using the normal and cancerous cells of the Basal, Breast and Cervical cells. The geometrical parameters are fixed to \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\), \({\text{h}}=150\,\mathrm{nm}\),\({\text{s}}=500\,\mathrm{nm}, {{\text{r}}}_{1}=400\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\)
Figure 7 shows the wavelength dependent confinement losses of the quasi-TM core mode at different RG constant(s) in both, normal and cancerous cell. The initial value of s is fixed to 500 nm. The value of s is varied by \(\pm\) 10% of its original value to show its effect on the sensing characteristics. In this study, other geometrical parameters are fixed to \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}, {{\text{r}}}_{1}=400\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\). As may be seen in Fig. 7, s variation has a slight effect on the resonance wavelength and hence on the sensitivity of the proposed biosensor. The maximum sensitivity values are obtained at s = 500 nm and equal to 18,100 nm/RIU, 18,500 nm/RIU, and 18,250 nm/RIU for Basel, Breast, and Cervical cells, respectively. The obtained results reveal that the sensitivity increases slightly by increasing s for all selected cancerous cells. Therefore, s is fixed at 500 nm to keep the fabrication feasibility of the proposed biosensor and obtain the highest sensitivity.
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different rectangular grating space (s) using the normal and cancerous cells of the Basal, Breast and Cervical cells. The geometrical parameters are fixed to \(\Lambda =2.3 \mu m\), \(d=3 \mu m\), \(h=150 nm\),\(L=450 nm, {r}_{1}=400 nm\),\({r}_{2}=800 nm\), and \({G}_{n}=5\)
Next, the effect of variation of the radii \({{\text{r}}}_{1}\) (radius of the small holes) and \({{\text{r}}}_{2}\) (radius of the large holes), as shown in Fig. 1, on the behavior of the proposed biosensor is studied and the results are shown in Figs. 8 and 9, respectively. It is worth mentioning that in this study, other geometrical parameters are fixed to \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\), while s is fixed at 550 nm. Here, the values of \({{\text{r}}}_{1}\) and \({{\text{r}}}_{2}\) are scaled up and down by 50 nm from their initial values (i.e., \({{\text{r}}}_{1}= 400\,\mathrm{nm}\) and \({{\text{r}}}_{2}= 800\,\mathrm{nm}\)). As the small air holes directly surround the core region, \({{\text{r}}}_{1}\) is expected to have a direct effect on the core mode and accordingly on the coupling with the SPM. However, \({{\text{r}}}_{2}\) is expected to have a slight effect on the mode confinement and the coupling operation. Increasing \({{\text{r}}}_{1}\) would increase the mode confinement and accordingly the neff of the core mode increases. Thus, the coupling between the core mode and SPM would occur at shorter wavelengths. Figures 8 and 9 depict the variation of the losses of the quasi-TM core mode with the operating wavelength for the 3 studied cell types at different values of \({{\text{r}}}_{1}\) and \({{\text{r}}}_{2}\), respectively. As may be seen in these figures, changing \({{\text{r}}}_{1}\) has larger effect on the resonance wavelength and sensitivity. However, as depicted in Fig. 9, varying \({{\text{r}}}_{2}\) induces a slight effect on the resonance wavelength and sensitivity when compared to the effect of variation in \({{\text{r}}}_{1}\). Additionally, increasing the two radii \({{\text{r}}}_{1}\) and \({{\text{r}}}_{2}\) shifts the resonance peaks to shorter wavelengths. It is worth mentioning that the maximum obtained sensitivities are 19,500 nm/RIU, 20,285 nm/RIU, and 19,791 nm/RIU for Basel, Breast, and Cervical cell, respectively with \({{\text{r}}}_{1}=350\,\mathrm{nm}\). On the other hand, at \({{\text{r}}}_{2}=750\,\mathrm{nm}\), sensitivities of 19,750 nm/RIU, 20,428 nm/RIU, and 20,041 nm/RIU for Basel, Breast, and Cervical cell, respectively are obtained. Therefore, \({{\text{r}}}_{1}\) and \({{\text{r}}}_{2}\) are fixed to 350 nm and 750 nm to keep the sensitivity at its highest possible level and keep the fabrication feasibility of the proposed biosensor.
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different small air hole radius \({{\text{r}}}_{1}\) using the normal and cancerous cells of the Basal, Breast and Cervical cells. The geometrical parameters are fixed to \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\), \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}\),\({{\text{r}}}_{2}=800\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\)
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different large air hole radius \({r}_{2}\), using the normal and cancerous cells of the Basal, Breast and Cervical cells. The geometrical parameters are fixed to \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\), \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}\),\({{\text{r}}}_{1}=350\,\mathrm{nm}\), and \({{\text{G}}}_{{\text{n}}}=5\)
The effect of grating elements on the sensor performance is then studied. In this investigation, the geometrical parameters are taken as \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}\), \(\mathrm{s }= 550\,\mathrm{nm}\), \({{\text{r}}}_{1}\) \(= 350\,\mathrm{nm}\), and \({{\text{r}}}_{1}\) \(= 750\,\mathrm{nm}\). Figure 10 depicts the variation of the losses of the quasi-TM core mode with the operating wavelength at different \({{\text{G}}}_{{\text{n}}}\) for the 3 studied cell types. The number of RG elements \({{\text{G}}}_{{\text{n}}}\) is varied between 3 different values of 3, 5, and 7. As may be seen in Fig. 10, the variation of \({{\text{G}}}_{{\text{n}}}\) has approximately no effect on the resonance wavelength. Thus, \({{\text{G}}}_{{\text{n}}}\) is fixed to 5.
Variation of the confinement losses of the quasi-TM core mode with the wavelength at different grating number (Gn) using the normal and cancerous cells of the Basal, Breast and Cervical cell. The geometrical parameters are fixed to \(\Lambda =2.3\,\upmu\mathrm{m}\), \({\text{d}}=3\,\upmu\mathrm{m}\), \({\text{h}}=150\,\mathrm{nm}\),\({\text{L}}=450\,\mathrm{nm}\),\({{\text{r}}}_{1}=350\,\mathrm{nm}\),\({\mathrm{and r}}_{2}=750\,\mathrm{nm}\)
As a result of the performed parametric analysis, the optimum parameters of the proposed biosensor are selected to obtain the highest sensitivities in the 3 studied cell types and keep the fabrication process feasible. The optimum parameters are summarized in Table 3.
The variation in resonance wavelength according to the change in the analyte RI is then studied and analyzed to prove the linear-like behavior of the proposed biosensor. Here, variation of resonance wavelength with respect to the analyte refractive index for normal and cancerous cells, is plotted in Fig. 11. It is worth mentioning that current study is performed by utilizing the geometrical parameters depicted in Table 3. From the plotted graphs in Fig. 11, it may be seen that there is an inversely linear relationship between the resonance wavelength and the analyte refractive index in all studied cases. It is worth mentioning that when na increases, the field confinement in the analyte region is enhanced and accordingly, the neff of the SPM increases. In this situation, to achieve the phase matching between the core mode and the SPM, Lam must be decreased. Additionally, the average squared correlation coefficient (\({{\text{R}}}^{2}\)) (Surdo and Barillaro 2015) of the line best fitting for all curves shown in Fig. 11 is high of 0.9999. Further, the obtained curves show satisfactory linear responses which verify the high efficiency and feasibility of the proposed biosensor.”
A tolerance analysis of the suggested design is performed to study the effect of variation in different geometrical parameters on the performance of the proposed structure. These small variations that may occur through the fabrication process can change the obtained sensitivity and the overall behavior of the proposed biosensor. In each study, only one geometrical parameter is studied while all other geometrical parameters are kept at their optimized values as summarized in Table 4. As may be seen from this table, the variations in geometrical parameters by \(\pm 10\mathrm{ \%}\) induce very small changes in the optical sensitivities that not exceed 5.9% of the optimum sensitivity value. It is worth noting that only the parameter \({{\text{r}}}_{1}\), that is the radius of the first ring of air holes directly surround the core region, has a large effect on the sensitivity value as depicted in Table 4. This is because \({{\text{r}}}_{1}\) directly affect the resonance wavelength. Thus, the obtained sensitivity value differs. However, in all cases, the obtained sensitivity is quite large and approaches 20,000 nm/RIU which is still higher than those reported in Ayyanar et al. (2018), Jabin et al. (2019), Mishra et al. (2020), Vijayalakshmi and Manimegalai (2021), Yasli (2021).
To reveal the significance of the reported biosensor, a comprehensive comparison is made with those reported in literature as summarized in Table 5 in terms of the basic platform/structure, the maximum gained sensitivity and studied cell type(s). From this table, it may be seen that the proposed PCF biosensor with RRG structure has higher sensitivity than that of those reported in Ayyanar et al. (2018), Jabin et al. (2019), Mishra et al. (2020), Vijayalakshmi and Manimegalai (2021) and Yasli (2021). Further, the obtained results for the three studied cell types; Basal, Breast, and Cervical readily identify sensing needs and justify the effectiveness of the biosensor.
4 Conclusion
A highly sensitive SPR D-shaped PCF configuration with rectangular grating has been proposed to sense three types of cancerous cells (Basal, Breast, and Cervical). FVFEM is utilized to analyze the confinement losses, resonance conditions, and wavelength shifts. An SPR optical fiber refractive index transducer was designed and simulated by utilizing rhodium plasmonic material which claim the effectiveness of the biosensor. Depending on the sample, the change in refractive index leads to a shift in the loss spectrum. Different structural parameters are optimized for maximum sensitivities of 19,750 nm/RIU, 20,428 nm/RIU, and 20,041 nm/RIU for Basel, Breast and Cervical cancer cells, respectively. Numerical results obtained in this manuscript are much higher comparable with previously presented articles in the literature (Ayyanar et al. 2018; Jabin et al. 2019; Mishra et al. 2020; Vijayalakshmi and Manimegalai 2021; Yasli 2021). Compared to the Long Period Fiber Gratings (LPFG) (coated and in TAP configuration), the reported biosensor based on the short Period Fiber Grating has small size and good light interaction with the analyte sample. The proposed SPR-PCF biosensor gives much better sensitivity compared to (Dey et al. 2022; Ling et al. 2019). Regarding future work, new plasmonic materials will be tested to enhance the operation of the presented sensor in terms of sensitivity. Additionally, the proposed D-shaped PCF can be extended to the fabrication phase to perform characterization and test the overall performance.
Data availability
The data will be available upon request.
References
Abarca-Quesada, D.A., Vargas-Quirós, C.E., Vargas-Castro, W.E.: Physical properties of rhodium retrieved from modeling its dielectric function by a simulated annealing approach. OSA Contin. 4, 3233–3253 (2021)
Adachi, S.: Handbook on Optical Constants of Metals, The: In Tables and Figures. World Scientific, Singapore (2012)
Ahmadivand, A., Sinha, R., Kaya, S., Pala, N.: Rhodium plasmonics for deep-ultraviolet bio-chemical sensing. Plasmonics 11, 839–849 (2016)
Akowuah, E.K., Gorman, T., Ademgil, H., Haxha, S., Robinson, G.K., Oliver, J.V.: Numerical analysis of a photonic crystal fiber for biosensing applications. IEEE J. Quantum Electron. 48, 1403–1410 (2012)
Al Mahfuz, M., Hossain, M.A., Haque, E., Hai, N.H., Namihira, Y., Ahmed, F.: Dual-core photonic crystal fiber-based plasmonic RI sensor in the visible to near-IR operating band. IEEE Sens. J. 20, 7692–7700 (2020)
Alrayk, Y.K.A., Younis, B.M., El Deeb, W.S., Hameed, M.F.O., Obayya, S.S.A.: MIR optical modulator based on silicon-on-calcium fluoride platform with VO2 material. Opt. Quantum Electron. 53, 1–16 (2021)
Amouzad Mahdiraji, G., Chow, D.M., Sandoghchi, S.R., Amirkhan, F., Dermosesian, E., Yeo, K.S., Kakaei, Z., Ghomeishi, M., Poh, S.Y., Yu Gang, S.: Challenges and solutions in fabrication of silica-based photonic crystal fibers: an experimental study. Fiber Integr. Opt. 33, 85–104 (2014)
An, G., Li, S., An, Y., Wang, H., Zhang, X.: Glucose sensor realized with photonic crystal fiber-based Sagnac interferometer. Opt. Commun. 405, 143–146 (2017)
André, R.M., Pevec, S., Becker, M., Dellith, J., Rothhardt, M., Marques, M.B., Donlagic, D., Bartelt, H., Frazão, O.: Focused ion beam post-processing of optical fiber Fabry-Perot cavities for sensing applications. Opt. Express 22, 13102–13108 (2014)
Ayyanar, N., Raja, G.T., Sharma, M., Kumar, D.S.: Photonic crystal fiber-based refractive index sensor for early detection of cancer. IEEE Sens. J. 18, 7093–7099 (2018)
Azab, M.Y., Hameed, M.F.O., Mahdiraji, G.A., Adikan, F.R.M., Obayya, S.S.A.: Experimental and numerical characterization of a D-shaped PCF refractive index sensor. Opt. Quantum Electron. 54, 846 (2022)
Birks, T.A., Knight, J.C., Russell, P.S.J.: Endlessly single-mode photonic crystal fiber. Opt. Lett. 22, 961–963 (1997). https://doi.org/10.1364/OL.22.000961
Cai, L., Zhang, S., Hu, H.: A compact photonic crystal micro-cavity on a single-mode lithium niobate photonic wire. J. Opt. 18, 35801 (2016)
Cerrina, F.: X-ray imaging: applications to patterning and lithography. J. Phys. D Appl. Phys. 33, R103 (2000)
Chaudhary, S., Amphawan, A.: Solid core PCF-based mode selector for MDM-Ro-FSO transmission systems. Photonic Netw. Commun. 36, 263–271 (2018)
Chen, M.-Y., Yu, R.-J., Zhao, A.-P.: Highly birefringent rectangular lattice photonic crystal fibres. J. Opt. A Pure Appl. Opt. 6, 997 (2004)
Czerczak, S., Gromiec, J.P., Pałaszewska-Tkacz, A., Świdwińska-Gajewska, A.: Nickel, ruthenium, rhodium, palladium, osmium, and platinum. Patty’s Toxicol. 6th ed., pp. 653–768. John Wiley Sons Ltd. New York (2012)
Dey, T.K., Tombelli, S., Roy, A., Biswas, P., Giannetti, A., Basumallick, N., Baldini, F., Bandyopadhyay, S., Trono, C.: Sensitivity analysis of sidelobes of the lowest order cladding mode of long period fiber gratings at turn around point. Sensors 22, 2965 (2022)
Dey, T.K., Tombelli, S., Biswas, P., Giannetti, A., Basumallick, N., Baldini, F., Bandyopadhyay, S., Trono, C.: Realization of enhanced evanescent field long period fiber grating near turn around point for label-free immunosensing. In: Proceedings, p. 9. MDPI (2020)
El-Saeed, A.H., Khalil, A.E., Hameed, M.F.O., Azab, M.Y., Obayya, S.S.A.: Highly sensitive SPR PCF biosensors based on Ag/TiN and Ag/ZrN configurations. Opt. Quantum Electron. 51, 1–18 (2019)
Filippov, V.N., Starodumov, A.N., Minkovich, V.P., Lecona, F.G.P.: Fiber sensor for simultaneous measurement of voltage and temperature. IEEE Photonics Technol. Lett. 12, 1543–1545 (2000)
Goto, H., Komata, K., Minami, S.: Impact of Pd–Rh interaction on the performance of three-way catalysts. SAE Technical Paper (2014)
Gutiérrez, Y., Alcaraz de la osa, R., Ortiz, D., Saiz, J.M., González, F., Moreno, F.: Plasmonics in the ultraviolet with aluminum, gallium, magnesium and rhodium. Appl. Sci. 8, 64 (2018)
Hameed, M.F.O., Obayya, S.S.A.: Polarization rotator based on soft glass photonic crystal fiber with liquid crystal core. J. Light. Technol. 29(18), 2725–2731 (2011)
Hameed, M.F.O., Obayya, S.S.A., Wiltshire, R.J.: Beam propagation analysis of polarization rotation in soft glass nematic liquid crystal photonic crystal fibers. IEEE Photonics Technol. Lett. 22(3), 188–190 (2010)
Hameed, M.F.O., Heikal, A.M., Obayya, S.S.A.: Novel passive polarization rotator based on spiral photonic crystal fiber. IEEE Photonics Technol. Lett. 25(16), 1578–1581 (2013)
Hansen, T.P., Broeng, J., Libori, S.E.B., Knudsen, E., Bjarklev, A., Jensen, J.R., Simonsen, H.: Highly birefringent index-guiding photonic crystal fibers. IEEE Photonics Technol. Lett. 13, 588–590 (2001). https://doi.org/10.1109/68.924030
Hiza, O.Y.M., Younis, B.M., Areed, N.F.F., Hameed, M.F.O., Obayya, S.S.A.: Compact TE-pass polarizer based on silicon-on-insulator platform with bimetallic rhodium–silver grating. Opt. Quantum Electron. 55, 493 (2023)
Hong, R., Sun, H., Li, D., Yang, W., Fan, K., Liu, C., Dong, L., Wang, G.: A review of biosensors for detecting tumor markers in breast cancer. Life. 12, 342 (2022)
Jabin, M.A., Ahmed, K., Rana, M.J., Paul, B.K., Islam, M., Vigneswaran, D., Uddin, M.S.: Surface plasmon resonance based titanium coated biosensor for cancer cell detection. IEEE Photonics J. 11, 1–10 (2019)
Jabir, J.N., Areebi, N.A.: High sensitively of double-core surface plasmon resonance biosensor based on photonic crystal fiber. Opt. Quantum Electron. 54, 626 (2022)
Ju, J., Jin, W., Demokan, M.S.: Properties of a highly birefringent photonic crystal fiber. IEEE Photonics Technol. Lett. 15, 1375–1377 (2003)
Kawano, K., Kitoh, T.: Introduction to Optical Waveguide Analysis: Solving Maxwell’s Equation and the Schrödinger Equation. John Wiley & Sons, Hoboken (2004)
Kim, S.E., Kim, B.H., Lee, C.G., Lee, S., Oh, K., Kee, C.-S.: Elliptical defected core photonic crystal fiber with high birefringence and negative flattened dispersion. Opt. Express 20, 1385–1391 (2012)
Knight, J.C., Birks, T.A., Cregan, R.F., Russell, P.S.J., de Sandro, P.D.: Large mode area photonic crystal fibre. Electron. Lett. 34, 1347–1348 (1998). https://doi.org/10.1049/el:19980965
Kolodziej, C.M., Maynard, H.D.: Electron-beam lithography for patterning biomolecules at the micron and nanometer scale. Chem. Mater. 24, 774–780 (2012)
Koshiba, M., Tsuji, Y.: Curvilinear hybrid edge/nodal elements with triangular shape for guided-wave problems. J. Light. Technol. 18, 737–743 (2000)
Li, Y., Luo, D.: Fabrication and application of 1D micro-cavity film made by cholesteric liquid crystal and reactive mesogen. Opt. Mater. Express 6, 691–696 (2016)
Li, T., Dong, X., Chan, C.C., Ni, K., Zhang, S., Shum, P.P.: Humidity sensor with a PVA-coated photonic crystal fiber interferometer. IEEE Sens. J. 13, 2214–2216 (2013a)
Li, W., Cheng, H., Xia, M., Yang, K.: An experimental study of pH optical sensor using a section of no-core fiber. Sensors Actuators A Phys. 199, 260–264 (2013b)
Li, A., Cheng, J., Yow, A.P., Srivastava, R., Wong, D.W.K., Tey, H.L., Liu, J.: Automated basal cell carcinoma detection in high-definition optical coherence tomography. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 2885–2888. IEEE (2016)
Ling, Q., Gu, Z., Jiang, X., Gao, K.: Design of long period fiber grating surrounding refractive index sensor based on mode transition near phase-matching turning point. Opt. Commun. 439, 187–192 (2019)
Meyers, H.P., Myers, H.P.: Introductory Solid State Physics. CRC Press, Boca Raton (1997)
Mishra, A.K., Mishra, S.K.: Gas sensing in Kretschmann configuration utilizing bi-metallic layer of rhodium–Silver in visible region. Sensors Actuators B Chem. 237, 969–973 (2016)
Mishra, G.P., Kumar, D., Chaudhary, V.S., Murmu, G.: Cancer cell detection by a heart-shaped dual-core photonic crystal fiber sensor. Appl. Opt. 59, 10321–10329 (2020)
Momota, M.R., Hasan, M.R.: Hollow-core silver coated photonic crystal fiber plasmonic sensor. Opt. Mater. (amst) 76, 287–294 (2018)
Morshed, M., Hassan, M.I., Roy, T.K., Uddin, M.S., Razzak, S.M.A.: Microstructure core photonic crystal fiber for gas sensing applications. Appl. Opt. 54, 8637–8643 (2015)
Nayak, C.: Numerical study of temperature and pressure effect on one dimensional random photonic crystal used as biosensors in the detection of breast cancer cells. Phys. Scr. 98, 25503 (2023)
Niciński, K., Krajczewski, J., Kudelski, A., Witkowska, E., Trzcińska-Danielewicz, J., Girstun, A., Kamińska, A.: Detection of circulating tumor cells in blood by shell-isolated nanoparticle–enhanced Raman spectroscopy (SHINERS) in microfluidic device. Sci. Rep. 9, 9267 (2019)
Obayya, S.S.A., Somasiri, N., Rahman, B.M.A., Grattan, K.T.: V: Full vectorial finite element modeling of novel polarization rotators. Opt. Quantum Electron. 35, 297–312 (2003)
Paul, A.K., Habib, M.S., Hai, N.H., Razzak, S.M.A.: An air-core photonic crystal fiber based plasmonic sensor for high refractive index sensing. Opt. Commun. 464, 125556 (2020)
Peng, L., Shi, F., Zhou, G., Ge, S., Hou, Z., Xia, C.: A surface plasmon biosensor based on a D-shaped microstructured optical fiber with rectangular lattice. IEEE Photonics J. 7, 1–9 (2015)
Quero, G., Crescitelli, A., Paladino, D., Consales, M., Buosciolo, A., Giordano, M., Cutolo, A., Cusano, A.: Evanescent wave long-period fiber grating within D-shaped optical fibers for high sensitivity refractive index detection. Sens. Actuators B Chem. 152, 196–205 (2011)
Rabee, A.S.H., Hameed, M.F.O., Heikal, A.M., Obayya, S.S.A.: Highly sensitive photonic crystal fiber gas sensor. Optik 188, 78–86 (2019)
Rajeswari, D., Revathi, A.A.: Highly sensitive SPR-based PCF bio sensor for plasma cell detection in human blood for the detection of early stage cancer. Optik (stuttg.) 258, 168897 (2022)
Ribaut, C., Loyez, M., Larrieu, J.-C., Chevineau, S., Lambert, P., Remmelink, M., Wattiez, R., Caucheteur, C.: Cancer biomarker sensing using packaged plasmonic optical fiber gratings: towards in vivo diagnosis. Biosens. Bioelectron. 92, 449–456 (2017)
Sani, M.H., Ghanbari, A., Saghaei, H.: High-sensitivity biosensor for simultaneous detection of cancer and diabetes using photonic crystal microstructure. Opt. Quantum Electron. 54, 1–14 (2022)
Sharan, P., Bharadwaj, S.M., Gudagunti, F.D., Deshmukh, P.: Design and modelling of photonic sensor for cancer cell detection. IMPETUS, (2014)
Sharma, P., Sharan, P., Deshmukh, P.: A photonic crystal sensor for analysis and detection of cancer cells. In: 2015 International Conference on Pervasive Computing (ICPC), pp. 1–5. IEEE (2015)
Shu, X., Zhang, L., Bennion, I.: Sensitivity characteristics of long-period fiber gratings. J. Light. Technol. 20, 255 (2002)
Soylemez, S., Udum, Y.A., Kesik, M., Hızlıateş, C.G., Ergun, Y., Toppare, L.: Electrochemical and optical properties of a conducting polymer and its use in a novel biosensor for the detection of cholesterol. Sens. Actuators B Chem. 212, 425–433 (2015)
Sun, D., Guo, T., Ran, Y., Huang, Y., Guan, B.-O.: In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 61, 541–546 (2014)
Sun, Z.-F., Chang, Y., Xia, N.: Recent development of nanomaterials-based cytosensors for the detection of circulating tumor cells. Biosensors 11, 281 (2021)
Surdo, S., Barillaro, G.: On the performance of label-free biosensors based on vertical one-dimensional photonic crystal resonant cavities. Opt. Express 23, 9192–9201 (2015)
Tong, K., Wang, F., Wang, M., Dang, P., Wang, Y., Sun, J.: D-shaped photonic crystal fiber biosensor based on silver-graphene. Optik (stuttg). 168, 467–474 (2018)
Usman, F., Dennis, J.O., Aljameel, A.I., Ali, M.K.M., Aldaghri, O., Ibnaouf, K.H., Zango, Z.U., Beygisangchin, M., Alsadig, A., Meriaudeau, F.: Plasmonic biosensors for the detection of lung cancer biomarkers: a review. Chemosensors. 9, 326 (2021)
Verma, P., Kumar, A., Jindal, P.: Machine learning approach for SPR based photonic crystal fiber sensor for breast cancer cells detection. In: 2022 IEEE 7th Forum on Research and Technologies for Society and Industry Innovation (RTSI). pp. 7–12. IEEE (2022)
Vigneswaran, D., Ayyanar, N., Sharma, M., Sumathi, M., Rajan, M., Porsezian, K.: Salinity sensor using photonic crystal fiber. Sens. Actuators A Phys. 269, 22–28 (2018)
Vijayalakshmi, D., Manimegalai, C.T.: Breast cancer cell recognition using high sensitivity photonic crystal fiber based plasmonic sensor. In: 2021 8th International Conference on Signal Processing and Integrated Networks (SPIN), pp. 1064–1068. IEEE (2021)
Virk, J.K., Das, S., Kaler, R.S., Singh, H., Kundu, T.: D-shape optical fiber probe dimension optimization for LSPR based bio-sensor. Opt. Fiber Technol. 71, 102930 (2022)
Wadsworth, W.J., Percival, R.M., Bouwmans, G., Knight, J.C., Russell, P.S.J.: High power air-clad photonic crystal fibre laser. Opt. Express 11, 48–53 (2003)
Weaver, J.H., Olson, C.G., Lynch, D.W.: Optical investigation of the electronic structure of bulk Rh and Ir. Phys. Rev. B 15, 4115 (1977)
World Health Organization, https://www.who.int/health-topics/cancer#tab=tab_1
Yan, H.-T., Liu, Q., Ming, Y., Luo, W., Chen, Y., Lu, Y.: Metallic grating on a D-shaped fiber for refractive index sensing. IEEE Photonics J. 5, 4800706 (2013)
Yaroslavsky, A.N., Patel, R., Salomatina, E., Li, C., Lin, C., Al-Arashi, M., Neel, V.: High-contrast mapping of basal cell carcinomas. Opt. Lett. 37, 644–646 (2012)
Yaru, S.H.I., Zhang, Y., Wei, W., Xiong, T., Dandan, D., Cheng, S.U.N.: A study of plasmonic properties of a rhodium I-shaped nanomaterial. Mater. Sci. 29, 154 (2023)
Yasli, A.: Cancer detection with surface plasmon resonance-based photonic crystal fiber biosensor. Plasmonics 16, 1605–1612 (2021)
Zettsu, N., McLellan, J.M., Wiley, B., Yin, Y., Li, Z., Xia, Y.: Synthesis, stability, and surface plasmonic properties of rhodium multipods, and their use as substrates for surface-enhanced raman scattering. Angew. Chemie. 118, 1310–1314 (2006)
Zhang, T., Zheng, Y., Wang, C., Mu, Z., Liu, Y., Lin, J.: A review of photonic crystal fiber sensor applications for different physical quantities. Appl. Spectrosc. Rev. 53, 486–502 (2018)
Zhang, X., Gutiérrez, Y., Li, P., Barreda, Á.I., Watson, A.M., de la Osa, R.A., Finkelstein, G., González, F., Ortiz, D., Saiz, J.M.: Plasmonics in the UV range with rhodium nanocubes. In: Nanophotonics VI, pp. 158–163. SPIE (2016)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors acknowledge the financial support from the Science, Technology & Innovation Funding Authority (STIFA) in Egypt under the project (ID: 45702).
Author information
Authors and Affiliations
Contributions
MFOH and BMY have proposed the idea. NAE has done the simulations of the reported sensor. All authors have contributed to the analysis, discussion, writing and revision of the paper.
Corresponding authors
Ethics declarations
Competing Interest
The authors would like to clarify that there are no financial/non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethical Approval
The authors declare that there are no conflicts of interest related to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elmahdy, N.A., Hameed, M.F.O., Obayya, S.S.A. et al. Highly sensitive plasmonic-grating PCF biosensor for cancer cell detection. Opt Quant Electron 56, 688 (2024). https://doi.org/10.1007/s11082-023-06250-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-06250-1