Abstract
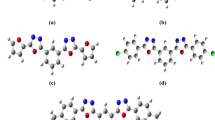
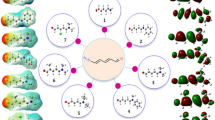
The electronic charge delocalization in organic compounds with a donor–acceptor system allows them to exhibit excellent nonlinear optical characteristics. From the perspective of conjugation, mono-carbonyl curcuminoids also have a fascinating skeleton. Interesting chemical structures of the (3E,5E)-3,5-dibenzylidene-piperidin-4-one derivatives motivate us to perform density functional theory-based studies. In investigating the derivates, geometric parameters, highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energies, and nonlinear optical (NLO) parameters calculations were performed using B3LYP/6-311++G(d,p) level of theory. Root mean square error (RMSE) calculations revealed excellent agreement between calculated and experimental parameters. The molecular stability of chalcone derivatives was investigated using molecular electrostatic potential analyses, which were also used to gain information regarding atomic charges. Calculated HOMO–LUMO energies showed that charge transfer interactions occur within the molecules. The HOMO and LUMO energies were used to compute hardness, softness, ionization potential, and electrophilicity. The \(\lambda_{max}\) values of the investigated compounds are greater than that of the reference compound. Among all other derivatives, B4 has the highest amplitude of static linear polarizability (\({\upalpha }_{tot}\)) and first total hyperpolarizability (\({\upbeta }_{tot}\)) values at 101.15 and 554.11 × 10–30 esu, respectively. Compelling NLO findings reveal that bis-chalcones-based derivatives could substantially contribute to NLO technology.









Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbas, H., Shkir, M., AlFaify, S.: Density functional study of spectroscopy, electronic structure, linear and nonlinear optical properties of l-proline lithium chloride and l-proline lithium bromide monohydrate: for laser applications. Arab. J. Chem. 12, 2336–2346 (2019). https://doi.org/10.1016/j.arabjc.2015.02.011
Adant, C., Dupuis, M., Bredas, J.L.: Ab initio study of the nonlinear optical properties of urea: electron correlation and dispersion effects. Int. J. Quantum Chem. 56, 497–507 (1995). https://doi.org/10.1002/qua.560560853
Amiri, S.S., Makarem, S., Ahmar, H., Ashenagar, S.: Theoretical studies and spectroscopic characterization of novel 4-methyl-5-((5-phenyl-1, 3, 4-oxadiazol-2-yl) thio) benzene-1, 2-diol. J. Mol. Struct. 1119, 18–24 (2016)
Ammasi, A., Iruthayaraj, R., Munusamy, A.P., Shkir, M.: Molecular engineering on D–π–A organic dyes with flavone-based different acceptors for highly efficient dye-sensitized solar cells using experimental and computational study. J. Mol. Model. 29, 45 (2023a). https://doi.org/10.1007/s00894-023-05445-3
Ammasi, A., Iruthayaraj, R., Munusamy, A.P., Shkir, M., Vellingiri, B., Minnam Reddy, V.R., Kim, W.K.: Molecular screening of different π-linker-based organic dyes for optoelectronic applications: quantum chemical study. J. Electron. Mater. 52, 3774–3785 (2023b). https://doi.org/10.1007/s11664-023-10338-5
Andrews, D.L., Bradshaw, D.S., Coles, M.M.: Perturbation theory and the two-level approximation: a corollary and critique. Chem. Phys. Lett. 503, 153–156 (2011). https://doi.org/10.1016/j.cplett.2010.12.055
Arunkumar, A., Anbarasan, P.M.: Optoelectronic properties of a simple metal-free organic sensitizer with different spacer groups: quantum chemical assessments. J. Electron. Mater. 48, 1522–1530 (2019). https://doi.org/10.1007/s11664-018-06912-x
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998). https://doi.org/10.1021/jp9716997
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993). https://doi.org/10.1063/1.464913
Bernstein, J., Davis, R.E., Shimoni, L., Chang, N.-L.: Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. EngL. 34, 1555–1573 (1995). https://doi.org/10.1002/anie.199515551
Bosshard, C., Hulliger, J., Florsheimer, M., Gunter, P.: Organic Nonlinear Optical Materials. CRC Press, Boca Raton (2001)
Cai, Z.-B., Bai, L., Pan, Y.-L., Ma, F.-F., Li, S.-L., Tian, Y.-P.: Multipolar symmetric and asymmetric N–heterocyclic compounds with efficient two−photon absorption. J. Photochem. Photobiol. A Chem. 346, 194–205 (2017)
Chai, J.-D., Head-Gordon, M.: Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008)
Champagne, B., Kirtman, B.: Evaluation of alternative sum-over-states expressions for the first hyperpolarizability of push-pull π-conjugated systems. J. Chem. Phys. (2006). https://doi.org/10.1063/1.2206181
Chattaraj, P.K., Maiti, B., Sarkar, U.: Philicity: a unified treatment of chemical reactivity and selectivity. J. Phys. Chem. A 107, 4973–4975 (2003)
Chen, R., Xu, K., Wang, G., Zhang, Z., Cao, L., Teng, B.: Theoretical and experimental investigation of a pyridinium-based NLO crystal 4-N, N-dimethylamino-4′-N′-methyl-stilbazolium 3,4-dimethoxysulfonate. Optik (stuttg). 257, 168830 (2022). https://doi.org/10.1016/j.ijleo.2022.168830
Chidan Kumar, C.S., Quah, C.K., Balachandran, V., Fun, H.-K., Asiri, A.M., Chandraju, S., Karabacak, M.: Synthesis, single crystal structure, spectroscopic characterization and molecular properties of (2E)-3-(2,6-dichlorophenyl)-1-(3,4-dimethoxyphenyl)prop-2-en-1-one. J. Mol. Struct. 1116, 135–145 (2016). https://doi.org/10.1016/j.molstruc.2016.02.089
Christodoulides, D.N., Khoo, I.C., Salamo, G.J., Stegeman, G.I., Van Stryland, E.W.: Nonlinear refraction and absorption: mechanisms and magnitudes. Adv. Opt. Photonics. 2, 60–200 (2010)
Cigan, M., Donovalová, J., Szöcs, V., Gaspar, J., Jakusová, K., Gaplovsky, A.: 7-(Dimethylamino) coumarin-3-carbaldehyde and its phenylsemicarbazone: TICT excited state modulation, fluorescent H-aggregates, and preferential solvation. J. Phys. Chem. A 117, 4870–4883 (2013)
Cisse, L., Djande, A., Capo-Chichi, M., Delatre, F., Saba, A., Tine, A., Aaron, J.-J.: Revisiting the photophysical properties and excited singlet-state dipole moments of several coumarin derivatives. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 79, 428–436 (2011)
Cossi, M., Rega, N., Scalmani, G., Barone, V.: Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 24, 669–681 (2003). https://doi.org/10.1002/jcc.10189
Duan, Y., Geng, Y., Li, H., Jin, J., Wu, Y., Su, Z.: Theoretical characterization and design of small molecule donor material containing naphthodithiophene central unit for efficient organic solar cells. J. Comput. Chem. 34, 1611–1619 (2013)
Erickson, M.A., Beels, M.T., Biaggio, I.: Optimum conjugation length in donor–acceptor molecules for third-order nonlinear optics. JOSA B 33, E130–E142 (2016)
Eryanti, Y., Herlina, T., Zamri, A., Shiono, Y., Awang, K., Halim, S.N.A., Supratman, U.: N-benzyl-(3E,5E)-3,5-bis(2-hydroxybenzylidene)-4-piperidone. Molbank 2015, 5–8 (2015). https://doi.org/10.3390/M852
Fejer, M.M.: Nonlinear optical frequency conversion. Phys. Today 47, 25–33 (1994)
Feng, L., Maddox, M.M., Alam, M.Z., Tsutsumi, L.S., Narula, G., Bruhn, D.F., Wu, X., Sandhaus, S., Lee, R.B., Simmons, C.J.: Synthesis, structure–activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives. J. Med. Chem. 57, 8398–8420 (2014)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian-09 Revision D.01. Gaussian, Inc., Wallingford (2013)
Gandhimathi, R., Vinitha, G., Dhanasekaran, R.: Effect of substituent position on the properties of chalcone isomer single crystals. J. Cryst. Process Technol. 3, 148–155 (2013)
Garmire, E.: Nonlinear optics in daily life. Opt. Express. 21, 30532–30544 (2013)
Ghouili, A., Dusek, M., Petricek, V., Ayed, T.B., Hassen, R.B.: Synthesis, crystal structure and spectral characteristics of highly fluorescent chalcone-based coumarin in solution and in polymer matrix. J. Phys. Chem. Solids. 75, 188–193 (2014). https://doi.org/10.1016/j.jpcs.2013.09.011
Gunasekaran, S., Balaji, R.A., Kumeresan, S., Anand, G., Srinivasan, S.: Experimental and theoretical investigations of spectroscopic properties of N-acetyl-5-methoxytryptamine. Can. J. Anal. Sci. Spectrosc. 53, 149–162 (2008)
Hagar, M., Ahmed, H.A., Aljohani, G., Alhaddad, O.A.: Investigation of some antiviral N-heterocycles as COVID 19 drug: molecular docking and DFT calculations. Int. J. Mol. Sci. 21, 3922 (2020). https://doi.org/10.3390/ijms21113922
Hrobarik, P., Sigmundova, I., Zahradnik, P., Kasak, P., Arion, V., Franz, E., Clays, K.: Molecular engineering of benzothiazolium salts with large quadratic hyperpolarizabilities: can auxiliary electron-withdrawing groups enhance nonlinear optical responses? J. Phys. Chem. C 114, 22289–22302 (2010)
Hussein, H.A., Fadhil, G.F.: Theoretical Investigation of para amino-dichloro chalcone isomers. Part II: a DFT structure-stability study of the FMO and NLO properties. ACS Omega 8, 4937–4953 (2023). https://doi.org/10.1021/acsomega.2c07148
Hutama, A.S., Huang, H., Kurniawan, Y.S.: Investigation of the chemical and optical properties of halogen-substituted N-methyl-4-piperidone curcumin analogs by density functional theory calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 221, 117152 (2019). https://doi.org/10.1016/j.saa.2019.117152
Irfan, A., Al-Sehemi, A.G., Assiri, M.A., Mumtaz, M.W.: Exploring the electronic, optical and charge transfer properties of acene-based organic semiconductor materials. Bull. Mater. Sci. 42, 1–7 (2019a)
Irfan, A., Chaudhry, A.R., Al-Sehemi, A.G., Assiri, M.A., Hussain, A.: Charge carrier and optoelectronic properties of phenylimidazo [1, 5-a] pyridine-containing small molecules at molecular and solid-state bulk scales. Comput. Mater. Sci. 170, 109179 (2019b)
Ito, M., Mikami, N.: Multiphoton spectroscopy. Appl. Spectrosc. Rev. 16, 299–352 (1980)
Ivanova, B.B., Spiteller, M.: Noncentrosymmetric crystals with marked nonlinear optical properties. J. Phys. Chem. A 114, 5099–5103 (2010)
Kuebler, S.M., Rumi, M.: NONLINEAR OPTICS, APPLICATIONS | Three-Dimensional Microfabrication. In: Encyclopedia of Modern Optics. pp. 189–206. Elsevier (2005)
Kusumawati, Y., Ivansyah, A.L., Ali, B.T.I., Kurnia, K.A., Hutama, A.S., Fansuri, H.: Photophysical properties of ammonium, pyrrolidinium, piperidinium, imidazolium, and pyridinium as a guide to prepare ionic-organic hybrid materials. Heliyon 8, e09121 (2022). https://doi.org/10.1016/j.heliyon.2022.e09121
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988). https://doi.org/10.1103/PhysRevB.37.785
Lesar, A., Milošev, I.: Density functional study of the corrosion inhibition properties of 1, 2, 4-triazole and its amino derivatives. Chem. Phys. Lett. 483, 198–203 (2009)
Lifshitz, R., Arie, A., Bahabad, A.: Photonic quasicrystals for nonlinear optical frequency conversion. Phys. Rev. Lett. 95, 133901 (2005)
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). https://doi.org/10.1002/jcc.22885
Maidur, S.R., Patil, P.S., Ekbote, A., Chia, T.S., Quah, C.K.: Molecular structure, second-and third-order nonlinear optical properties and DFT studies of a novel non-centrosymmetric chalcone derivative:(2E)-3-(4-fluorophenyl)-1-(4-{[(1E)-(4-fluorophenyl) methylene] amino} phenyl) prop-2-en-1-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 184, 342–354 (2017)
Majumder, M., Misra, A.: Strategic design of thiophene-fused nickel dithiolene derivatives for efficient NLO response. Phys. Chem. Chem. Phys. 20, 19007–19016 (2018)
Marlina, L.A., Haryadi, W., Daengngern, R., Pranowo, H.D.: Molecular design of benzo[c][1,2,5]thiadiazole or thieno[3,4-d]pyridazine-based auxiliary acceptors through different anchoring groups in D–π–A–A framework: a DFT/TD-DFT study. J. Mol. Graph. Model. 113, 108148 (2022a). https://doi.org/10.1016/j.jmgm.2022.108148
Marlina, L.A., Haryadi, W., Pranowo, H.D.: Design of a D–π–A–A framework with various auxiliary acceptors on optoelectronic and charge transfer properties for efficient dyes in DSSCs: a DFT/TD-DFT study. J. Comput. Electron. 21, 361–377 (2022b). https://doi.org/10.1007/s10825-022-01851-7
Mary, Y.S., Panicker, C.Y., Anto, P.L., Sapnakumari, M., Narayana, B., Sarojini, B.K.: Molecular structure, FT-IR, NBO, HOMO and LUMO, MEP and first order hyperpolarizability of (2E)-1-(2, 4-Dichlorophenyl)-3-(3, 4, 5-trimethoxyphenyl) prop-2-en-1-one by HF and density functional methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 135, 81–92 (2015)
Matos, M.J., Vazquez-Rodriguez, S., Uriarte, E., Santana, L.: Potential pharmacological uses of chalcones: a patent review (from June 2011–2014). Expert Opin. Ther. Pat. 25, 351–366 (2015)
Miar, M., Shiroudi, A., Pourshamsian, K., Oliaey, A.R., Hatamjafari, F.: Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo [d] thiazole-2 (3 H)-imine and its para-substituted derivatives: Solvent and substituent effects. J. Chem. Res. 45, 147–158 (2021)
Mo, Y., Lin, Z., Wu, W., Zhang, Q.: Bond-distorted orbitals and effects of hybridization and resonance on C–C bond lengths. J. Phys. Chem. 100, 11569–11572 (1996). https://doi.org/10.1021/jp953433a
Morgante, P., Peverati, R.: The devil in the details: a tutorial review on some undervalued aspects of density functional theory calculations. Int. J. Quantum Chem. 120, e26332 (2020). https://doi.org/10.1002/qua.26332
Muhammad, S.: Symmetric vs. asymmetric: Which one is the better molecular configuration for achieving robust NLO response? J. Mol. Graph. Model. 114, 108209 (2022). https://doi.org/10.1016/j.jmgm.2022.108209
Muhammad, S., Al-Sehemi, A.G., Su, Z., Xu, H., Irfan, A., Chaudhry, A.R.: First principles study for the key electronic, optical and nonlinear optical properties of novel donor-acceptor chalcones. J. Mol. Graph. Model. 72, 58–69 (2017)
Muhammad, S., Nakano, M., Al-Sehemi, A.G., Irfan, A., Chaudhry, A.R., Tonami, T., Ito, S., Kishi, R., Kitagawa, Y.: Exploring the novel donor-nanotube archetype as an efficient third-order nonlinear optical material: asymmetric open-shell carbon nanotubes. Nanoscale 10, 16499–16507 (2018)
Muhammad, S., Sarwar, F., Bibi, S., Nadeem, R., Mushtaq, M.W., Al-Sehemi, A.G., Alarfaji, S.S., Hussain, S.: Insighting the functionally modified C60 fullerenes as an efficient nonlinear optical materials: aA quantum chemical study. Mater. Sci. Semicond. Process. 141, 106421 (2022)
Naik, V.S., Patil, P.S., Wong, Q.A., Quah, C.K., Gummagol, N.B., Jayanna, H.S.: Molecular structure, linear optical, second and third-order nonlinear optical properties of two non-centrosymmetric thiophene-chalcone derivatives. J. Mol. Struct. 1222, 128901 (2020)
Nakano, M., Fukuda, K., Champagne, B.: Third-order nonlinear optical properties of asymmetric non-alternant open-shell condensed-ring hydrocarbons: effects of diradical character, asymmetricity, and exchange interaction. J. Phys. Chem. C 120, 1193–1207 (2016)
Nesterov, V.N.: 3,5-Bis(4-methoxybenzylidene)-1-methyl-4-piperidone and 3,5-bis(4-methoxybenzylidene)-1-methyl-4-oxopiperidinium chloride: Potential biophotonic materials. Acta. Crystallogr. Sect. C Cryst. Struct. Commun. 60, 806–809 (2004). https://doi.org/10.1107/S0108270104022723
Oudar, J.L., Chemla, D.S.: Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 66, 2664–2668 (1976). https://doi.org/10.1063/1.434213
Oudar, J.L., Chemla, D.S.: Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 66, 2664–2668 (2008). https://doi.org/10.1063/1.434213
Panicker, C.Y., Varghese, H.T., Nayak, P.S., Narayana, B., Sarojini, B.K., Fun, H.K., War, J.A., Srivastava, S.K., Van Alsenoy, C.: Infrared spectrum, NBO, HOMO–LUMO, MEP and molecular docking studies (2E)-3-(3-nitrophenyl)-1-[4-piperidin-1-yl]prop-2-en-1-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 148, 18–28 (2015). https://doi.org/10.1016/j.saa.2015.03.065
Parr, R.G., Szentpály, L.V., Liu, S.: Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999)
Ponnusamy Munusamy, A., Ammasi, A., Shkir, M.: Computational analysis of carbazole-based newly efficient D–π–A organic spacer dye derivatives for dye-sensitized solar cells. Struct. Chem. 33, 1097–1107 (2022). https://doi.org/10.1007/s11224-021-01853-4
Prasad, P.N., Williams, D.J.: Introduction to Nonlinear Optical Effects in Molecules and Polymers. Wiley, New York (1991)
Prommin, C., Kerdpol, K., Saelee, T., Kungwan, N.: Effects of π-expansion, an additional hydroxyl group, and substitution on the excited state single and double proton transfer of 2-hydroxybenzaldehyde and its relative compounds: TD-DFT static and dynamic study. New J. Chem. 43, 19107–19119 (2019). https://doi.org/10.1039/c9nj05055h
Shao, Y., Mei, Y., Sundholm, D., Kaila, V.R.I.: Benchmarking the performance of time-dependent density functional theory methods on biochromophores. J. Chem. Theory Comput. 16, 587–600 (2020). https://doi.org/10.1021/acs.jctc.9b00823
Silva, D.L., Fonseca, R.D., Vivas, M.G., Ishow, E., Canuto, S., Mendonca, C.R., De Boni, L.: Experimental and theoretical investigation of the first-order hyperpolarizability of a class of triarylamine derivatives. J. Chem. Phys. 142, 064312 (2015). https://doi.org/10.1063/1.4906893
Sumithra, I.S., Jayashri, T.A., Krishnan, G.: X-ray diffraction, spectroscopic, thermal and surface morphological studies of gamma-irradiated diaquamalonatomanganese(II) (DMM). J. Radioanal. Nucl. Chem. 307, 835–842 (2016). https://doi.org/10.1007/s10967-015-4283-2
Sutradhar, T., Misra, A.: Theoretical study on the nonlinear optical property of boron nitride nanoclusters functionalized by electron donating and electron accepting groups. J. Phys. Chem. A. 125, 2436–2445 (2021)
Tahir, M.N., Khalid, M., Islam, A., Mashhadi, S.M.A., Braga, A.A.C.: Facile synthesis, single crystal analysis, and computational studies of sulfanilamide derivatives. J. Mol. Struct. 1127, 766–776 (2017)
Tamer, Ö., Avci, D., Atalay, Y.: Calculations of electronic structure and nonlinear optical parameters of 4-methoxybenzaldehyde-N-methyl-4-stilbazolium tosylate*. J. Appl. Spectrosc. 80, 971–982 (2014). https://doi.org/10.1007/s10812-014-9875-z
Tejkiran, P.J., Teja, M.S.B., Kumar, P.S.S., Sankar, P., Philip, R., Naveen, S., Lokanath, N.K., Rao, G.N.: DA–π–D Synthetic approach for thienyl chalcones–NLO–a structure activity study. J. Photochem. Photobiol. A Chem. 324, 33–39 (2016)
Thamarai, A., Vadamalar, R., Raja, M., Muthu, S., Narayana, B., Ramesh, P., Muhamed, R.R., Sevvanthi, S., Aayisha, S.: Molecular structure interpretation, spectroscopic (FT-IR, FT-Raman), electronic solvation (UV–Vis, HOMO-LUMO and NLO) properties and biological evaluation of (2E)-3-(biphenyl-4-yl)-1-(4-bromophenyl) prop-2-en-1-one: experimental and computational modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 226, 117609 (2020)
Thomas, A., Chitumalla, R.K., Puyad, A.L., Mohan, K.V., Jang, J.: Computational studies of hole/electron transport in positional isomers of linear oligo-thienoacenes: evaluation of internal reorganization energies using density functional theory. Comput. Theor. Chem. 1089, 59–67 (2016)
Tu, Y., Chai, K., Wu, J., Hu, Y., Shi, S., Yang, D., Yao, T.: A rational design to improve selective imaging of tau aggregates by constructing side substitution on N, N-dimethylaniline/quinoxaline D–π–A fluorescent probe. Sens. Actuators B Chem. 380, 133406 (2023). https://doi.org/10.1016/j.snb.2023.133406
Vazquez-Rodriguez, S., López, R.L., Matos, M.J., Armesto-Quintas, G., Serra, S., Uriarte, E., Santana, L., Borges, F., Crego, A.M., Santos, Y.: Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorg. Med. Chem. 23, 7045–7052 (2015)
Virkki, M., Tuominen, O., Forni, A., Saccone, M., Metrangolo, P., Resnati, G., Kauranen, M., Priimagi, A.: Halogen bonding enhances nonlinear optical response in poled supramolecular polymers. J. Mater. Chem. C 3, 3003–3006 (2015)
Walker, M., Harvey, A.J.A., Sen, A., Dessent, C.E.H.: Performance of M06, M06–2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 117, 12590–12600 (2013)
Wazzan, N., Irfan, A.: Exploring the optoelectronic and charge transport properties of pechmann dyes as efficient OLED materials. Optik (stuttg) 197, 163200 (2019)
Wei, H., Ruan, J., Zhang, X.: Coumarin–chalcone hybrids: promising agents with diverse pharmacological properties. RSC Adv. 6, 10846–10860 (2016)
Wykes, M., Milián-Medina, B., Gierschner, J.: Computational engineering of low bandgap copolymers. Front. Chem. 1, 35 (2013)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004). https://doi.org/10.1016/j.cplett.2004.06.011
Yoneda, K., Nakano, M., Fukuda, K., Matsui, H., Takamuku, S., Hirosaki, Y., Kubo, T., Kamada, K., Champagne, B.: Third-order nonlinear optical properties of one-dimensional open-shell molecular aggregates composed of phenalenyl radicals. Chem. Eur. J. 20, 11129–11136 (2014)
Yousif, A.A., Fadhil, G.F.: DFT of para methoxy dichlorochalcone isomers. Investigation of structure, conformation, FMO, charge, and NLO properties. Chem. Data Collect. 31, 100618 (2021)
Yushina, I.D., Masunov, A.E., Lopez, D., Dyakov, A.A., Bartashevich, E.: V: Toward first-principles design of organic nonlinear optical materials: Crystal structure prediction and halogen bonding impact on hyperpolarizabilities of 2-iodo-3-hydroxypyridine. Cryst. Growth Des. 18, 5069–5079 (2018)
Zhao, Y., Truhlar, D.G.: Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008). https://doi.org/10.1021/ar700111a
Zyss, J.: Molecular Nonlinear Optics: Materials, Physics, and Devices. Academic Press, Cambridge (2013)
Acknowledgements
LAM acknowledges postdoctoral funding from the Manajemen Talenta BRIN fellowship program 2023 No. 15/II/HK/2023. The calculations were partly performed at Mahameru High-Performance Computing, National Research and Innovation Agency (BRIN) and SymBaHCat Cluster belonging to the Symbah Foundation.
Funding
L.A.M. acknowledges funding from Manajemen Talenta BRIN fellowship program 2023 No. 15/II/HK/2023.
Author information
Authors and Affiliations
Contributions
LAM conceived the idea, led the investigation and methodology, and equally contributed to data curation, formal analysis, funding acquisition, and writing the original draft. ASH contributed equally to formal analysis, supervision, validation, and original draft writing. SNZ and MFP provided support in the investigation. WJS assisted in writing, reviewing, and editing the manuscript. WDS took the lead in supervision and equally contributed to reviewing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11082_2023_5383_MOESM1_ESM.pdf
Cartesian coordinate of optimized compounds, HOMO−LUMO map of the parent molecule, and hyperpolarizability tensor of the tested compounds. (PDF 359 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marlina, L.A., Hutama, A.S., Zanah, S.N. et al. Performance of density functional theory for calculating the electronic, static, and dynamic nonlinear optical properties of asymmetric (3E,5E)-3,5-dibenzylidene-piperidin-4-one derivatives. Opt Quant Electron 55, 1081 (2023). https://doi.org/10.1007/s11082-023-05383-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05383-7