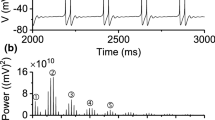
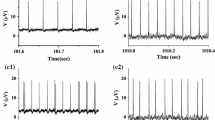
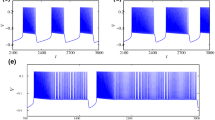
Abstract
Neuronal bursting patterns are nonlinear behaviors related to various brain functions and diseases. Bursting modulated by two slow variables manifest more complex dynamics than that with one slow variable, due to involved with more bifurcations of fast subsystem. The traditional dissection method or singular perturbation method often fails to analyze the bursting with two slow variables, for instance, a complex mixed-mode oscillation (MMO) bursting observed in the immature inner hair cell related to the development of auditory function. The MMO bursting exhibits alternation between a single spike with large amplitude and a pseudo-plateau burst behaving as fast burst with small oscillations. In the present paper, the MMO bursting is successfully analyzed using a novel dissection process. With two slow variables c and h regarded as bifurcation parameters of the fast subsystem, various dynamical behaviors and bifurcation curves are obtained. By identifying real intersections between the bursting trajectory and bifurcation curves in the (c, h, V) space instead of (c, h) plane, the pseudo-plateau burst is built relationships to the depolarization block and subcritical Hopf bifurcation of the fast subsystem, and the spike is associated with coexisting firing and limit point bifurcation of cycles. Interestingly, no quiescent state is identified to be related to the resting state or saddle-node bifurcation on an invariant cycle (SNIC), due to that c is not slow enough. As c becomes sufficiently slow, the bursting pattern is associated with the resting state and the SNIC. In addition, the bursting trajectory exhibits a narrow shape around an oblique line in the (c, h) plane, and the bifurcations along the line presents a simple and effective candidate to characterize the bursting. The results present effective process of dissection method and deep understanding of the MMO bursting, which are helpful for modulating the development of auditory functions related to the inner hair cell.














Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Johnson, S.L., Eckrich, T., Kuhn, S., Zampini, V., Franz, C., Ranatunga, K.M., Roberts, T.P., Masetto, S., Knipper, M., Kros, C.J., Marcotti, W.: Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat. Neurosci. 14(6), 711–717 (2011)
Iosub, R., Avitabile, D., Grant, L., Tsaneva-atanasova, K., Kennedy, H.J.: Calcium-induced calcium release during action potential firing in developing inner hair cells. Biophys. J. 108(5), 1003–1012 (2015)
Amro, R.M., Neiman, A.B.: Effect of bidirectional mechanoelectrical coupling on spontaneous oscillations and sensitivity in a model of hair cells. Phys. Rev. E 90, 052704 (2014)
Gu, H., Pan, B.: A four-dimensional neuronal model to describe the complex nonlinear dynamics observed in the firing patterns of a sciatic nerve chronic constriction injury model. Nonlinear Dyn. 81, 2107–2126 (2015)
Tsaneva-Atanasova, K., Sherman, A., Van Goor, F., Stojilkovic, S.S.: Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J. Neurophysiol. 98, 131–144 (2007)
Izhikevich, E.M., Desai, N.S., Walcott, E.C., Hoppensteadt, F.C.: Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26(3), 161–167 (2003)
Viala, D., Vidal, C., Freton, E.: Coordinated rhythmic bursting in respiratory and locomotor muscle nerves in the spinal rabbit. Neurosci. Lett. 11, 155–159 (1979)
Wang, X.J.: Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 90(3), 1195–1268 (2010)
Fan, D., Zheng, Y., Yang, Z., Wang, Q.: Improving control effects of absence seizures using single-pulse alternately resetting stimulation (SARS) of corticothalamic circuit. J. Appl. Math. Mech. 41(9), 1287–1302 (2020)
Gu, H., Pan, B.: Identification of neural firing patterns, frequency and temporal coding mechanisms in individual aortic baroreceptors. Front. Comput. Neurosci. 9, 108 (2015)
Duan, L., Liang, W., Ji, W., Xi, H.: Bifurcation patterns of bursting within pre-Bötzinger complex and their control. Int. J. Bifurcat. Chaos 30(13), 2050192 (2020)
Cui, Y., Yang, Y., Ni, Z., Dong, Y., Cai, G., Foncelle, A., Ma, S., Sang, K., Tang, S., Li, Y., Shen, Y., Berry, H., Wu, S., Hu, H.: Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018)
Yang, Y., Cui, Y., Sang, K., Dong, Y., Ni, Z., Ma, S., Hu, H.: Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018)
Teka, W., Tabak, J., Vo, T., Wechselberger, M., Bertram, R.: The dynamics underlying pseudo-plateau bursting in a pituitary cell model. J. Math. Neurosci. 1, 12 (2011)
Loppini, A., Pedersen, M.G.: Gap-junction coupling can prolong beta-cell burst period by an order of magnitude via phantom bursting. Chaos 28(6), 063111 (2018)
Liu, Y., Liu, S.: Canard-induced mixed-mode oscillations and bifurcation analysis in a reduced 3D pyramidal cell model. Nonlinear Dyn. 101, 531–567 (2020)
Liu, Y., Liu, S.: Characterizing mixed-mode oscillations shaped by canard and bifurcation structure in a three-dimensional cardiac cell model. Nonlinear Dyn. 103, 2881–2902 (2021)
Babola, T.A., Li, S., Gribizis, A., Lee, B.J., Issa, J.B., Wang, H.C., Crair, M.C., Bergles, D.E.: Homeostatic control of spontaneous activity in the developing auditory system. Neuron 99, 1–14 (2018)
Wang, H.C., Lin, C.C., Cheung, R., Zhang-Hooks, Y., Agarwal, A., Ellis-Davies, G., Rock, J., Bergles, D.E.: Spontaneous activity of cochlear hair cells triggered by fluid secretion mechanism in adjacent support cells. Cell 163, 1348–1359 (2015)
Ma, J.: Biophysical neurons, energy, and synapse controllability: a review. Zhejiang Univ. Sci. A 24(2), 109–129 (2023)
Guo, Y., Zhou, P., Yao, Z., Ma, J.: Biophysical mechanism of signal encoding in an auditory neuron. Nonlinear Dyn. 105, 3603–3614 (2021)
Cao, B., Gu, H., Bai, J., Wu, F.: Bifurcation and chaos of spontaneous oscillations of hair bundles in auditory hair cells. Int. J. Bifurcat. Chaos 31(4), 2130011 (2021)
Cao, B., Gu, H., Ma, K.: Complex dynamics of hair bundle of auditory nervous system (I): spontaneous oscillations and two cases of steady states. Cogn. Neurodyn. 16, 917–940 (2022)
Cao, B., Gu, H., Wang, R.: Complex dynamics of hair bundle of auditory nervous system (II): forced oscillations related to two cases of steady state. Cogn. Neurodyn. 16, 1163–1188 (2022)
Ermentrout, G.B., Terman, D.H.: Mathematical Foundations of Neuroscience. Springer, New York (2010)
Wang, Y., Rubin, J.E.: Multiple timescale mixed bursting dynamics in a respiratory neuron model. J. Comput. Neurosci. 41, 245–268 (2016)
Yang, Z., Lu, Q.: Bifurcation mechanisms of electrical bursting with different-time-scale slow variables. Int. J. Bifurcat. Chaos 21(5), 1407–1425 (2011)
Desroches, M., Guckenheimer, J., Krauskop, B., Kuehn, C., Osinga, H.M., Wechselberger, M.: Mixed-mode oscillations with multiple time scales. SIAM Rev. 54(2), 211–288 (2012)
Fenichel, N.: Geometric singular perturbation theory for ordinary differential equations. J. Differ. Equ. 31, 53–98 (1979)
Jones, C.K.R.T.: Geometric Singular Perturbation Theory. Dynamical Systems. Springer, New York (1995)
Rubin, J.E., Terman, D.: Geometric singular perturbation analysis of neuronal dynamics. Handb. Dyn. Syst. 2, 93–146 (2002)
Brøns, M., Krupa, M., Wechselberger, M.: Mixed mode oscillations due to the generalized Canard phenomenon. Field Inst. Commun. 49, 39–63 (2006)
Vo, T., Bertram, R., Tabak, J., Wechselberger, M.: Mixed mode oscillations as a mechanism for pseudo-plateau bursting. J. Comput. Neurosci. 28(3), 443–458 (2010)
Teka, W., Tabak, J., Bertram, R.: The relationship between two fast/slow analysis techniques for bursting oscillations. Chaos 22, 043117 (2012)
Baldemir, H., Avitabile, D., Tsaneva-atanasova, K.: Pseudo-plateau bursting and mixed-mode oscillations in a model of developing inner hair cells. Nonlinear Sci. Numer. Simul. 80, 104979 (2020)
Liu, Y., Liu, S., Lu, B., Kurths, J.: Mixed-mode oscillations for slow–fast perturbed systems. Phys. Scr. 96, 125258 (2021)
Lu, B., Liu, S., Jiang, X., Wang, J., Wang, X.: The mixed-mode oscillations in Av-Ron-Parnas-Segel model. Discrete Contin. Dyn. Syst. S 10(3), 487–504 (2017)
Rinzel, J.: Bursting Oscillations in an Excitable Membrane Model. Ordinary and Partial Differential Equations, Springer (1985)
Rinzel, J.: A formal classification of bursting mechanisms in excitable systems. In: Mathematical Topics in Population Biology, Morphogenesis, and Neurosciences. Lecture Notes in Biomathematics. Springer (1987)
Izhikevich, E.M.: Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting. MIT Press, London (2007)
Izhikevich, E.M.: Neural excitability, spiking and bursting. Int. J. Bifurcat. Chaos 10(6), 1171–1266 (2000)
Osinga, H.M., Sherman, A., Tsaneva-Atanasova, K.: Cross-currents between biology and mathematics: the codimension of pseudo-plateau bursting. Discrete Contin. Dyn. Syst. A 32(8), 2853–2877 (2012)
Yang, Z., Lu, Q., Li, L.: The genesis of period-adding bursting without bursting-chaos in the Chay model. Chaos Soliton Fract. 27, 87–95 (2006)
Cao, B., Wang, R., Gu, H., Li, Y.: Coherence resonance for neuronal bursting with spike undershoot. Cogn. Neurodyn. 15, 77–90 (2021)
Guan, L., Gu, H., Jia, Y.: Multiple coherence resonances evoked from bursting and the underlying bifurcation mechanism. Nonlinear Dyn. 100(4), 3645–3666 (2020)
Duan, L., Liu, J., Chen, X., Xiao, P., Zhao, Y.: Dynamics of in-phase and anti-phase bursting in the coupled pre-Bötzinger complex cells. Cogn. Neurodyn. 11, 91–97 (2017)
Han, X., Bi, Q., Zhang, C., Yu, Y.: Delayed bifurcations to repetitive spiking and classification of delay-induced bursting. Int. J. Bifurcat. Chaos 24(7), 1450098 (2014)
Wen, Q., Liu, S., Lu, B.: Firing patterns and bifurcation analysis of neurons under electromagnetic induction. Electron. Res. Arch. 29(5), 3205–3226 (2021)
Wouapi, M.K., Fotsin, B.H., Ngouonkadi, E.B.M., Kemwoue, F.F., Njitacke, Z.T.: Complex bifurcation analysis and synchronization optimal control for Hindmarsh–Rose neuron model under magnetic flow effect. Cogn. Neurodyn. 15, 315–347 (2021)
Cressman, J.R., Ullah, G., Ziburkus, J., Schiff, S.J., Barreto, E.: Erratum: The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J. Comput. Neurosci. 26, 159–170 (2009)
Li, Y., Gu, H., Jia, Y., Ma, K.: Fast–slow variable dissection with two slow variables related to calcium concentrations: a case study to bursting in a neural pacemaker model. Nonlinear Dyn. 107, 1223–1245 (2022)
Meng, P., Lu, Q., Wang, Q.: Dynamical analysis of bursting oscillations in the Chay–Keizer model with three time scales. Sci. China Technol. Sci. 54(8), 2024–2032 (2011)
Lü, Z., Chen, L., Duan, L.: Bifurcation analysis of mixed bursting in the pre-Bötzinger complex. Appl. Math. Model. 67, 234–251 (2019)
Lü, Z., Liu, M., Duan, L.: Dynamical analysis of dendritic mixed bursting within the pre-Bötzinger complex. Nonlinear Dyn. 103, 897–912 (2021)
Del Negro, C.A., Hsiao, C.F., Chandler, S.H., Garfinkel, A.: Evidence for a novel bursting mechanism in rodent trigeminal neurons. Biophys. J. 75, 174–182 (1998)
Ma, K., Gu, H., Zhao, Z.: Fast-slow variable dissection with two slow variables: a case study on bifurcations underlying bursting for seizure and spreading depression. Int. J. Bifurcat. Chaos 31(6), 2150096 (2021)
Szalai, R., Tsaneva-Atanasova, K., Homer, M.E., Champneys, A.R., Kennedy, H.J., Cooper, N.P.: Nonlinear models of development, amplification and compression in the mammalian cochlea. Phil. Trans. R. Soc. A 369, 4183–4204 (2011)
Johnson, S.L., Adelman, J.P., Marcotti, W.: Genetic deletion of SK2 channels in mouse inner hair cells prevents the developmental linearization in the \(\rm Ca^{2+} \) dependence of exocytosis. J. Physiol. 583(2), 631–646 (2007)
Marcotti, W., Johnson, S.L., Kros, J.: A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J. Physiol. 560(3), 691–708 (2004)
Marcotti, W., Johnson, S.L., Rüsch, A., Kros, C.J.: Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J. Physiol. 552(3), 743–761 (2003)
Environments, B.: Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. SIAM, Philadelphia (2002)
Dhooge, A., Govaerts, W., Kuznetsov, Y.A.: MATCONT: a MATLAB package for numerical bifurcation analysis of ODEs. ACM Trans. Math. Softw. 29(1), 141–164 (2003)
Funding
This article was funded by National Natural Science Foundation of China (11872276, 12072236).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was sponsored by the National Natural Science Foundation of China (Grant Numbers: 12072236, 11872276).
Appendix: Bifurcations of 3-dimensional fast subsystem fails to explain the bursting dynamics
Appendix: Bifurcations of 3-dimensional fast subsystem fails to explain the bursting dynamics
As expected, bifurcations of 3-dimensional fast subsystem fail to explain the bursting dynamics fails to explain the dynamics of the “\(1+4\)” burst. In the present Appendix, we show that the failure results and compared with the results of the main text. Such a comparison is helpful for comprehensive and deep understanding to the dynamics of “\(1+4\)” bursting
1.1 Fast subsystem
Similar to many nervous systems, the concentration of intracellular calcium ion c is the slow variable. Therefore, the dissection method with c taken as a single slow variable is used to identify the bifurcation mechanism underlying the MMO bursting “\(1+4\)”. Then, the fast subsystem is described as follows:
where \(I_{\textrm{Ca}} = g_{\textrm{Ca}}m_{\mathrm{\infty }}q_{\mathrm{\infty }}(V-V_{\textrm{Ca}})\) with \(q_{\mathrm{\infty }} =(1+\dfrac{c}{K_{\textrm{q}}})^{-1}\), and c is taken as bifurcation parameter.
1.2 Dynamics of the 3-dimenional fast subsystem
The bifurcations of equilibrium points and limit cycles with respect to c are illustrated in Fig. 15a. The lower branch (red solid) represents a stable node, which corresponds to the resting state with a lower membrane potential. The middle branch (black short dots) represents a saddle. The upper branch is divided into 2 parts by a subcritical Hopf bifurcation (\(\mathrm {HB_{\textrm{1}}}\)) occurring at c \(\approx \) 0.5763 \(\upmu \textrm{M}\). The red solid part left to the \(\mathrm {HB_{\textrm{1}}}\) represents a stable focus, which corresponds to the depolarization block with a higher membrane potential, and the part (black short dots) right to \(\mathrm {HB_{\textrm{1}}}\) denotes an unstable focus. The right turning point of the equilibrium point curve is a saddle-node (SN) bifurcation at c \(\approx \) 0.5948 \(\upmu \textrm{M}\), and the left turning point is a saddle-node bifurcation on an invariant cycle (SNIC) at c \(\approx \) 0.4683 \(\upmu \textrm{M}\). An unstable limit cycle (blue short dash line) appears via the Hopf bifurcation \(\mathrm {HB_{\textrm{1}}}\) and disappears via a limit point bifurcation of cycles (LPC) appearing at c \(\approx \) 0.3038 \(\upmu \textrm{M}\). Meanwhile, a stable limit cycle (green solid line) emerges via the LPC and terminates via the SNIC at c \(\approx \) 0.4683 \(\upmu \textrm{M}\), as shown in Fig. 15a. The spiking and resting state coexist between the LPC and SNIC points. In addition, the insert figure represents the enlargement around the SN point. A subcritical Hopf bifurcation (\(\mathrm {HB_{\textrm{2}}}\)) appears on the middle branch at c \(\approx \) 0.5946 \(\upmu \textrm{M}\). The unstable limit cycle (blue short dash curves) emerges from \(\mathrm {HB_{\textrm{2}}}\) and disappears via a saddle homoclinic orbit bifurcation (HC) at c \(\approx \) 0.5945 \(\upmu \textrm{M}\). Both the \(\mathrm {HB_{\textrm{2}}}\) and HC points have little influences on the dynamics of the MMO bursting “\(1+4\)”.
The dynamics of the MMO bursting “\(1+4\)” with c taken as a single slow variable. a The dynamics of the fast subsystem. The upper and lower red solid curves represent stable focus (depolarization block) and node (resting state), respectively, the black short dot curve of the upper branch denotes unstable focus. The black short dot curve of the middle branch represents unstable equilibrium. The SN, \(\mathrm {HB_{\textrm{1}}}\), and SNIC represent the corresponding bifurcations. The green solid (blue short dash) curve represents stable (unstable) limit cycle. The LPC represents limit point bifurcation of cycles. The insert represents the enlargement around the SN. The blue short dash curve represents unstable limit cycle, \(\mathrm {HB_{\textrm{2}}}\) represents Hopf bifurcation, and HC represents saddle homoclinic orbit bifurcation; b The bursting trajectory (black bold solid curve) and the critical phase points superimposed with panel (a). The phase points A1 and B1 (red circle) represent the extreme values of the single spike “1”, and A2 and B2 (red circle) represent the extreme values of the pseudo-plateau burst “4”. (Color figure online)
The difference of the bifurcations with respect to c between the two-dimensional fast subsystem [Eqs. (15–16)] at h \(\approx \) 0.9387 and the three-dimensional fast subsystem [Eqs. (A1–A3)] (thin curves, same as Fig. 15b). Black symbols HB and LPC are for the two-dimensional fast subsystem, green symbols \(\mathrm {HB_{\textrm{1}}}\), \(\mathrm {HB_{\textrm{2}}}\), HC, and LPC are for the 3-dimensional fast subsystem, and red SNIC is for both fast subsystems. (Color figure online)
1.3 No relationships between the bifurcations of the 3-dimensional fast subsystem and the bursting trajectory
The trajectory (black bold solid curve) of the MMO bursting “\(1+4\)” and four critical phase points A1, A2, B1, and B2 are superimposed to Fig. 15a to form Fig. 15b, and the direction of the trajectory is denoted by the arrows. As can be found from Fig. 3b, the amplitude (between point A1 and point B1) of the spike “1” is consistent with the amplitude of the stable limit cycle. But there is no obvious relationship between the bursting trajectory and the bifurcations, as addressed in the following three aspects.
-
(1)
The pseudo-plateau burst “4” has no relationship to the stable behaviors on the upper branch (red solid), which is different from the general bursting. For a general bursting, the burst always runs along the stable limit cycle [40].
-
(2)
No any part of the bursting trajectory is overlap with the lower branch, which is different from the general bursting. For a general bursting, the trajectory of quiescent state is overlap with the stable behavior on the lower branch. Although the two durations begging from the point B1 and from the point B2 look like the quiescent state of the bursting “\(1+4\)” in appearance, both durations are not overlap with the lower branch (lower red curve). Therefore, the two durations begging from the point B1 and from the point B2 should not be the quiescent state indeed.
-
(3)
The begging and ending phases of the spike “1” have no relationships to the bifurcations of the fast system such as the SNIC, LPC, or \(\mathrm {HB_{\textrm{1}}}\), which differs from the general bursting. For a general bursting, such phases should be related to the bifurcations.
1.4 Distinction of dynamics between the two-dimensional and three-dimensional fast subsystems
The bifurcations of the 3-dimensional fast subsystem [Eqs. (A1–A3)] fail to explain the “\(1+4\)” bursting, due to that h is regarded as fast variables. In Sect. 3.2, the bifurcations of the 2-dimensional fast subsystem [Eqs. (15–16)] can explain the dynamics of the bursting. The distinction of bifurcations between the 3-dimensional (thin curves) and 2-dimensional (bold curves) fast subsystems can be found from Fig. 16. The most important difference is that the upper branch (upper bold red) of the equilibrium (DB) for the 2-dimensional fast subsystem (h \(\approx \) 0.9387) becomes lower, compared with that (upper thin red) of the 3-dimensional fast subsystem. The pseudo-plateau burst “4” rotates around the lower membrane potential of the DB of the 2-dimensional fast system, while has no relationship to the DB of the 3-dimensional fast system. Then, the bifurcations of the two-dimensional fast subsystem modulated by both c and h are more effective to analyze the “\(1+4\)” bursting than those of the 3-dimensional fast system modulated by c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Gu, H., Hua, H. et al. Identifying bifurcations underlying a neuronal bursting of mixed-mode oscillations with two slow variables in inner hair cell. Nonlinear Dyn 111, 21957–21975 (2023). https://doi.org/10.1007/s11071-023-08980-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08980-z