Abstract
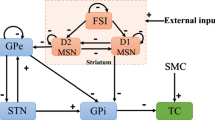
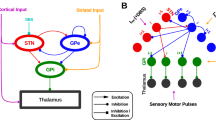
Dysfunction of basal ganglia is associated with the pathogenesis of Parkinson's disease including alteration of firing rate and excessive beta-band (13-30 Hz) synchronization activity. Neuronal heterogeneity enriches dynamics of external globus pallidus (GPe), especially showing significant differences in firing alterations under pathological state. The precise mechanism underlying these neural signatures remains elusive. To address this, we propose a subthalamopallidal network containing two classes of GPe neurons, calcium-binding protein parvalbumin (PV) and Lim homeobox (Lhx6) GPe. Our results show that Lhx6 GPe tends to rein in synchronous behavior and abnormal activity of PV GPe. Under the pathological condition, the alteration of synaptic coupling in a heterogenous pallidal network manifests itself as a direct increase of inhibitory input to PV GPe or an indirect elevation of Lhx6 GPe firing rate. These essentially enhance the inhibition of PV GPe, which results in beta-band synchronous bursting. The subthalamic nucleus (STN) is instrumental in stabilizing the spiking sequence of GPe neurons, inhibiting abnormal synchronous oscillations both in control and pathological conditions. In a dopamine-depleted state, the PV GPe-PV GPe pathway notably impacts the enhancement of beta rhythmic oscillations in STN-GPe circuit. Besides, the synaptic coupling in heterogenous pallidal and STN-GPe affect the propagation of abnormal rhythms in pallidal and subthalamopallidal networks, respectively. Our study highlights the pivotal role played by PV GPe in producing and amplifying pathological oscillatory behavior. Our results suggest that STN prevents abnormal oscillatory rhythm in GPe, providing a novel insight into the precise mechanism by which STN affects pallidal activity.








Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Yu, Y., Wang, X., Wang, Q., Wang, Q.: A review of computational modeling and deep brain stimulation: applications to Parkinson’s disease. Appl. Math. Mech.Engl. Ed. 41, 1747–1768 (2020). https://doi.org/10.1007/s10483-020-2689-9
Albin, R.L., Young, A.B., Penney, J.B.: The functional anatomy of basal ganglia disorders. Trend Neurosci. 12, 366–375 (1989). https://doi.org/10.1016/0166-2236(89)90074-X
Bergman, H., Feingold, A., Nini, A., Raz, A., Slovin, H., Abeles, M., Vaadia, E.: Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trend Neurosci. (1998). https://doi.org/10.1016/0166-2236(89)90074-X
Rubchinsky, L.L., Park, C., Worth, R.M.: Intermittent neural synchronization in parkinson’s disease. Nonlinear Dyn. 68, 329–346 (2012). https://doi.org/10.1007/s11071-011-0223-z
Mallet, N., Ballion, B., Le Moine, C., Gonon, F.: Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci. 26, 3875–3884 (2006). https://doi.org/10.1523/JNEUROSCI.4439-05.2006
Parker, J.G., Marshall, J.D., Ahanonu, B., Wu, Y.-W., Kim, T.H., Grewe, B.F., Zhang, Y., Li, J.Z., Ding, J.B., Ehlers, M.D., Schnitzer, M.J.: Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557, 177–182 (2018). https://doi.org/10.1038/s41586-018-0090-6
Kravitz, A.V., Freeze, B.S., Parker, P.R.L., Kay, K., Thwin, M.T., Deisseroth, K., Kreitzer, A.C.: Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626 (2010). https://doi.org/10.1038/nature09159
Stein, E., Bar-Gad, I.: β oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol. 245, 52–59 (2013). https://doi.org/10.1016/j.expneurol.2012.07.023
Brown, P.: Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. 18, 357–363 (2003). https://doi.org/10.1002/mds.10358
Weinberger, M., Mahant, N., Hutchison, W.D., Lozano, A.M., Moro, E., Hodaie, M., Lang, A.E., Dostrovsky, J.O.: Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J. Neurophysiol. 96, 3248–3256 (2006). https://doi.org/10.1152/jn.00697.2006
Khawaldeh, S., Tinkhauser, G., Torrecillos, F., He, S., Foltynie, T., Limousin, P., Zrinzo, L., Oswal, A., Quinn, A.J., Vidaurre, D., Tan, H., Litvak, V., Kühn, A., Woolrich, M., Brown, P.: Balance between competing spectral states in subthalamic nucleus is linked to motor impairment in Parkinson’s disease. Brain (2022). https://doi.org/10.1093/brain/awab264
Mallet, N., Pogosyan, A., Sharott, A., Csicsvari, J., Bolam, J.P., Brown, P., Magill, P.J.: Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. (2008). https://doi.org/10.1523/JNEUROSCI.0123-08.2008
Plenz, D., Kital, S.T.: A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400, 677–682 (1999). https://doi.org/10.1038/23281
Terman, D., Rubin, J.E., Yew, A.C., Wilson, C.J.: Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J. Neurosci. 22, 2963–2976 (2002). https://doi.org/10.1523/jneurosci.22-07-02963.2002
Park, C., Rubchinsky, L.L.: Potential mechanisms for imperfect synchronization in parkinsonian basal ganglia. PLoS ONE (2012). https://doi.org/10.1371/journal.pone.0051530
Mallet, N., Micklem, B.R., Henny, P., Brown, M.T., Williams, C., Bolam, J.P., Nakamura, K.C., Magill, P.J.: Dichotomous organization of the external globus pallidus. Neuron 74, 1075–1086 (2012). https://doi.org/10.1016/j.neuron.2012.04.027
Foster, N.N., Barry, J., Korobkova, L., Garcia, L., Gao, L., Becerra, M., Sherafat, Y., Peng, B., Li, X., Choi, J.-H., Gou, L., Zingg, B., Azam, S., Lo, D., Khanjani, N., Zhang, B., Stanis, J., Bowman, I., Cotter, K., Cao, C., Yamashita, S., Tugangui, A., Li, A., Jiang, T., Jia, X., Feng, Z., Aquino, S., Mun, H.-S., Zhu, M., Santarelli, A., Benavidez, N.L., Song, M., Dan, G., Fayzullina, M., Ustrell, S., Boesen, T., Johnson, D.L., Xu, H., Bienkowski, M.S., Yang, X.W., Gong, H., Levine, M.S., Wickersham, I., Luo, Q., Hahn, J.D., Lim, B.K., Zhang, L.I., Cepeda, C., Hintiryan, H., Dong, H.-W.: The mouse cortico–basal ganglia–thalamic network. Nature 598, 188–194 (2021). https://doi.org/10.1038/s41586-021-03993-3
Yu, Y., Wang, Q.: Oscillation dynamics in an extended model of thalamic-basal ganglia. Nonlinear Dyn. 98, 1065–1080 (2019). https://doi.org/10.1007/s11071-019-05249-2
Corbit, V.L., Whalen, T.C., Zitelli, K.T., Crilly, S.Y., Rubin, J.E., Gittis, A.H.: Pallidostriatal projections promote oscillations in a dopamine-depleted biophysical network model. J. Neurosci. 36, 5556–5571 (2016). https://doi.org/10.1523/jneurosci.0339-16.2016
McElvain, L.E., Chen, Y., Moore, J.D., Brigidi, G.S., Bloodgood, B.L., Lim, B.K., Costa, R.M., Kleinfeld, D.: Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron 109, 1721-1738.e4 (2021). https://doi.org/10.1016/j.neuron.2021.03.017
Mallet, N., Pogosyan, A., Marton, L.F., Bolam, J.P., Brown, P., Magill, P.J.: Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci. 28, 14245–14258 (2008). https://doi.org/10.1523/jneurosci.4199-08.2008
Cui, Q., Pamukcu, A., Cherian, S., Chang, I.Y.M., Berceau, B.L., Xenias, H.S., Higgs, M.H., Rajamanickam, S., Chen, Y., Du, X., Zhang, Y., McMorrow, H., Abecassis, Z.A., Boca, S.M., Justice, N.J., Wilson, C.J., Chan, C.S.: Dissociable roles of Pallidal neuron subtypes in regulating motor patterns. J. Neurosci. 41, 4036–4059 (2021). https://doi.org/10.1523/jneurosci.2210-20.2021
Abrahao, K.P., Lovinger, D.M.: Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice: classification of pallidal neurons. J. Physiol. 596, 4219–4235 (2018). https://doi.org/10.1113/jp276079
Dong, J., Hawes, S., Wu, J., Le, W., Cai, H.: Connectivity and functionality of the globus pallidus externa under normal conditions and Parkinson’s disease. Front. Neural Circuits. (2021). https://doi.org/10.3389/fncir.2021.645287
Kita, H., Kita, T.: Number, origins, and chemical types of rat pallidostriatal projection neurons. J. Comp. Neurol. 437, 438–448 (2001). https://doi.org/10.1002/cne.1294
Mastro, K.J., Bouchard, R.S., Holt, H.A.K., Gittis, A.H.: Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 34, 2087–2099 (2014). https://doi.org/10.1523/jneurosci.4646-13.2014
Evans, R.C., Twedell, E.L., Zhu, M., Ascencio, J., Zhang, R., Khaliq, Z.M.: Functional Dissection of Basal Ganglia Inhibitory Inputs onto Substantia Nigra Dopaminergic Neurons. Cell Rep. (2020). https://doi.org/10.1016/j.celrep.2020.108156
Oh, Y.-M., Karube, F., Takahashi, S., Kobayashi, K., Takada, M., Uchigashima, M., Watanabe, M., Nishizawa, K., Kobayashi, K., Fujiyama, F.: Using a novel PV-Cre rat model to characterize pallidonigral cells and their terminations. Brain Struct. Funct. 222, 2359–2378 (2017). https://doi.org/10.1007/s00429-016-1346-2
Abecassis, Z.A., Berceau, B.L., Win, P.H., García, D., Xenias, H.S., Cui, Q., Pamukcu, A., Cherian, S., Hernández, V.M., Chon, U., Lim, B.K., Kim, Y., Justice, N.J., Awatramani, R., Hooks, B.M., Gerfen, C.R., Boca, S.M., Chan, C.S. Npas1+ -Nkx2.1+ Neurons are an integral part of the cortico-pallido-cortical loop. J. Neurosci. 40, 743–768 (2020). https://doi.org/10.1523/jneurosci.1199-19.2019
Glajch, K.E., Kelver, D.A., Hegeman, D.J., Cui, Q., Xenias, H.S., Augustine, E.C., Hernandez, V.M., Verma, N., Huang, T.Y., Luo, M., Justice, N.J., Chan, C.S.: Npas1+ pallidal neurons target striatal projection neurons. J. Neurosci. 36, 5472–5488 (2016). https://doi.org/10.1523/jneurosci.1720-15.2016
Aristieta, A., Barresi, M., Azizpour Lindi, S., Barrière, G., Courtand, G., de la Crompe, B., Guilhemsang, L., Gauthier, S., Fioramonti, S., Baufreton, J., Mallet, N.P.: A disynaptic circuit in the globus pallidus controls locomotion inhibition. Curr. Biol. 31, 707-721.e7 (2021). https://doi.org/10.1016/j.cub.2020.11.019
Pamukcu, A., Cui, Q., Xenias, H.S., Berceau, B.L., Augustine, E.C., Fan, I., Chalasani, S., Hantman, A.W., Lerner, T.N., Boca, S.M., Chan, C.S.: Parvalbumin+ and Npas1+ pallidal neurons have distinct circuit topology and function. J. Neurosci. 40, 7855–7876 (2020). https://doi.org/10.1523/jneurosci.0361-20.2020
Jeon, H., Lee, H., Kwon, D.-H., Kim, J., Tanaka-Yamamoto, K., Yook, J.S., Feng, L., Park, H.R., Lim, Y.H., Cho, Z.-H., Paek, S.H., Kim, J. Topographic connectivity and cellular profiling reveal detailed input pathways and functionally distinct cell types in the subthalamic nucleus. Cell Rep. 38, 110439 (2022). https://doi.org/10.1016/j.celrep.2022.110439
Saunders, A., Huang, K.W., Sabatini, B.L. Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PLoS ONE. 11, e0149798 (2016). https://doi.org/10.1371/journal.pone.0149798
Ketzef, M., Silberberg, G.: Differential synaptic input to external globus pallidus neuronal subpopulations in vivo. Neuron 109, 516-529.e4 (2021). https://doi.org/10.1016/j.neuron.2020.11.006
Johansson, Y., Ketzef, M. Sensory processing in external globus pallidus neurons. Cell Rep. 42, 111952 (2023). https://doi.org/10.1016/j.celrep.2022.111952
Mastro, K.J., Zitelli, K.T., Willard, A.M., Leblanc, K.H., Kravitz, A.V., Gittis, A.H. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci. 20, (2017). https://doi.org/10.1038/nn.4559
Spix, T.A., Nanivadekar, S., Toong, N., Kaplow, I.M., Isett, B.R., Goksen, Y., Pfenning, A.R., Gittis, A.H. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science. 374, 201–206 (2021). https://doi.org/10.1126/science.abi7852
Yu, Y., Hao, Y., Wang, Q.: Model-based optimized phase-deviation deep brain stimulation for Parkinson ’s disease. Neural Netw. 122, 308–319 (2020). https://doi.org/10.1016/j.neunet.2019.11.001
Rubin, J.E., Terman, D.: High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J. Comput. Neurosci. 16, 211–235 (2004). https://doi.org/10.1023/b:jcns.0000025686.47117.67
Pirini, M., Rocchi, L., Sensi, M., Chiari, L. A computational modelling approach to investigate different targets in deep brain stimulation for Parkinson’s disease. J. Comput. Neurosci. 26, 91–107 (2009). https://doi.org/10.1007/s10827-008-0100-z
Yu, Y., Han, F., Wang, Q.: Exploring phase–amplitude coupling from primary motor cortex-basal ganglia–thalamus network model. Neural Netw. 153, 130–141 (2022). https://doi.org/10.1016/j.neunet.2022.05.027
de la Crompe, B., Aristieta, A., Leblois, A., Elsherbiny, S., Boraud, T., Mallet, N.P.: The globus pallidus orchestrates abnormal network dynamics in a model of Parkinsonism. Nat. Commun. 11, 1570 (2020). https://doi.org/10.1038/s41467-020-15352-3
Kovaleski, R.F., Callahan, J.W., Chazalon, M., Wokosin, D.L., Baufreton, J., Bevan, M.D.: Dysregulation of external globus pallidus-subthalamic nucleus network dynamics in parkinsonian mice during cortical slow-wave activity and activation. J. Physiol. 598, 1897–1927 (2020). https://doi.org/10.1113/jp279232
Hasegawa, T., Chiken, S., Kobayashi, K., Nambu, A. Subthalamic nucleus stabilizes movements by reducing neural spike variability in monkey basal ganglia. Nat. Commun. 13, 1–15 (2022). https://doi.org/10.1038/s41467-022-29750-2
Wang, X., Yu, Y., Han, F., Wang, Q. Beta-band bursting activity in computational model of heterogeneous external globus pallidus circuits. Commun. Nonlinear Sci. Numerical Simul. 110, 106388 (2022). https://doi.org/10.1016/j.cnsns.2022.106388
Gast, R., Gong, R., Schmidt, H., Meijer, H.G.E., Knösche, T.R.: On the role of arkypallidal and prototypical neurons for phase transitions in the external pallidum. J. Neurosci. 41, 6673–6683 (2021). https://doi.org/10.1523/JNEUROSCI.0094-21.2021
So, R.Q., Kent, A.R., Grill, W.M.: Relative contributions of local cell and passing fiber activation and silencing to changes in thalamic fidelity during deep brain stimulation and lesioning: a computational modeling study. J. Comput. Neurosci. 32, 499–519 (2012). https://doi.org/10.1007/s10827-011-0366-4
Brown, P., Williams, D.: Basal ganglia local field potential activity: Character and functional significance in the human. Clin. Neurophysiol. 116, 2510–2519 (2005). https://doi.org/10.1016/j.clinph.2005.05.009
Priori, A., Foffani, G., Rossi, L., Marceglia, S.: Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp. Neurol. 245, 77–86 (2013). https://doi.org/10.1016/j.expneurol.2012.09.013
Zhu, Y., Wang, J., Chang, S., Li, H., Deng, B., Liu, C.: Adaptive parameter modulation of deep brain stimulation in a computational model of basal ganglia–thalamic network. Nonlinear Dyn. 106, 945–958 (2021). https://doi.org/10.1007/s11071-021-06833-1
Parasuram, H., Nair, B., D’Angelo, E., Hines, M., Naldi, G., Diwakar, S. Computational modeling of single neuron extracellular electric potentials and network local field potentials using LFPsim. Front. Comput. Neurosci. 10, (2016). https://doi.org/10.3389/fncom.2016.00065
Einevoll, G.T., Kayser, C., Logothetis, N.K., Panzeri, S.: Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci. 14, 770–785 (2013). https://doi.org/10.1038/nrn3599
Buzsáki, G., Anastassiou, C.A., Koch, C.: The origin of extracellular fields and currents—EEG, ECoG LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012). https://doi.org/10.1038/nrn3241
Su, F., Wang, J., Niu, S., Li, H., Deng, B., Liu, C., Wei, X.: Nonlinear predictive control for adaptive adjustments of deep brain stimulation parameters in basal ganglia–thalamic network. Neural Netw. 98, 283–295 (2018). https://doi.org/10.1016/j.neunet.2017.12.001
Mazzoni, A., Lindén, H., Cuntz, H., Lansner, A., Panzeri, S., Einevoll, G.T. Computing the local field potential (LFP) from Integrate-and-fire network models. PLOS Comput. Biol. 11, e1004584 (2015). https://doi.org/10.1371/journal.pcbi.1004584
Martínez-Cañada, P., Ness, T.V., Einevoll, G.T., Fellin, T., Panzeri, S. Computation of the electroencephalogram (EEG) from network models of point neurons. PLOS Comput. Biol. 17, e1008893 (2021). https://doi.org/10.1371/journal.pcbi.1008893
Deister, C.A., Dodla, R., Barraza, D., Kita, H., Wilson, C.J.: Firing rate and pattern heterogeneity in the globus pallidus arise from a single neuronal population. J. Neurophysiol. 109, 497–506 (2013). https://doi.org/10.1152/jn.00677.2012
Abdi, A., Mallet, N., Mohamed, F.Y., Sharott, A., Dodson, P.D., Nakamura, K.C., Suri, S., Avery, S.V., Larvin, J.T., Garas, F.N., Garas, S.N., Vinciati, F., Morin, S., Bezard, E., Baufreton, J., Magill, P.J.: Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 35, 6667–6688 (2015). https://doi.org/10.1523/jneurosci.4662-14.2015
Gunay, C., Edgerton, J.R., Jaeger, D.: Channel density distributions explain spiking variability in the globus pallidus: a combined physiology and computer simulation database approach. J. Neurosci. (2008). https://doi.org/10.1523/JNEUROSCI.4198-07.2008
Moolchand, P., Jones, S.R., Frank, M.J.: Biophysical and architectural mechanisms of subthalamic theta under response conflict. J. Neurosci. (2022). https://doi.org/10.1523/JNEUROSCI.2433-19.2022
Nevado-Holgado, A.J., Mallet, N., Magill, P.J., Bogacz, R.: Effective connectivity of the subthalamic nucleus–globus pallidus network during parkinsonian oscillations. J. Physiol. 592, 1429–1455 (2014). https://doi.org/10.1113/jphysiol.2013.259721
Fujiyama, F., Nakano, T., Matsuda, W., Furuta, T., Udagawa, J., Kaneko, T.: A single-neuron tracing study of arkypallidal and prototypic neurons in healthy rats. Brain Struct. Funct. 221, 4733–4740 (2016). https://doi.org/10.1007/s00429-015-1152-2
Chazalon, M., Paredes-Rodriguez, E., Morin, S., Martinez, A., Cristóvão-Ferreira, S., Vaz, S., Sebastiao, A., Panatier, A., Boué-Grabot, E., Miguelez, C., Baufreton, J.: GAT-3 dysfunction generates tonic inhibition in external globus pallidus neurons in parkinsonian rodents. Cell Rep. 23, 1678–1690 (2018). https://doi.org/10.1016/j.celrep.2018.04.014
Miguelez, C., Morin, S., Martinez, A., Goillandeau, M., Bezard, E., Bioulac, B., Baufreton, J.: Altered pallido-pallidal synaptic transmission leads to aberrant firing of globus pallidus neurons in a rat model of Parkinson’s disease: increase in pallidal recurrent inhibition in experimental parkinsonism. J. Physiol. 590, 5861–5875 (2012). https://doi.org/10.1113/jphysiol.2012.241331
Bevan, M.D., Wilson, C.J., Bolam, J.P., Magill, P.J.: Equilibrium potential of GABAA current and implications for rebound burst firing in rat subthalamic neurons in vitro. J. Neurophysiol. 83, 3169–3172 (2000). https://doi.org/10.1152/jn.2000.83.5.3169
Fan, K.Y., Baufreton, J., Surmeier, D.J., Chan, C.S., Bevan, M.D.: Proliferation of external globus pallidus-subthalamic nucleus synapses following degeneration of midbrain dopamine neurons. J. Neurosci. 32, 13718–13728 (2012). https://doi.org/10.1523/jneurosci.5750-11.2012
Yin, L., Han, F., Yu, Y., Wang, Q.: A computational network dynamical modeling for abnormal oscillation and deep brain stimulation control of obsessive–compulsive disorder. Cogn. Neurodyn. (2022). https://doi.org/10.1007/s11571-022-09858-3
Yu, Y., Fan, Y., Hou, S., Wang, Q.: Optogenetic stimulation of primary motor cortex regulates beta oscillations in the basal ganglia: a computational study. Commun. Nonlinear Sci. Numer. Simul. (2023). https://doi.org/10.1016/j.cnsns.2022.106918
Lindahl, M., Kamali Sarvestani, I., Ekeberg, Ö., Kotaleski, J.H.: Signal enhancement in the output stage of the basal ganglia by synaptic short-term plasticity in the direct, indirect, and hyperdirect pathways. Front. Comput. Neurosci. (2013). https://doi.org/10.3389/fncom.2013.00076
Kumar, A., Prakash, A., Mehmet Baskonus, H.: The epidemic COVID-19 model via Caputo-Fabrizio fractional operator. Waves Random Complex Media (2022). https://doi.org/10.1080/17455030.2022.2075954
Sabir, Z., Umer, M., Raja, M.A.Z., Fathurrochman, I., Hasan, H.: Design of morlet wavelet neural network to solve the non-linear influenza disease system. Appl. Math. Nonlinear Sci. (2022). https://doi.org/10.2478/amns.2021.2.00120
Trejos, D.Y., Valverde, J.C., Venturino, E.: Dynamics of infectious diseases: a review of the main biological aspects and their mathematical translation. Appl. Math. Nonlinear Sci. 7, 1–26 (2022). https://doi.org/10.2478/amns.2021.1.00012
Ahn, S., Zauber, S.E., Worth, R.M., Rubchinsky, L.L.: Synchronized beta-band oscillations in a model of the globus pallidus-subthalamic nucleus network under external input. Front. Comput. Neurosci (2016). https://doi.org/10.3389/fncom.2016.00134
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grants Nos. 11932003, 12272092 and 12202027).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors designed, performed the research and analyzed the data as well as wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Yu, Y., Han, F. et al. Dynamical mechanism of parkinsonian beta oscillation in a heterogenous subthalamopallidal network. Nonlinear Dyn 111, 10505–10527 (2023). https://doi.org/10.1007/s11071-023-08381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08381-2