Abstract
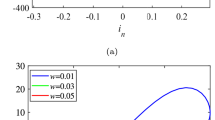
The environmental temperature plays a crucial role in determining the behavior of any dynamical system. In particular, living organisms are characterized by a specific value of temperature, which ensures their normal functioning. In this paper, the effect of temperature on signal transmission of a pacemaker neuron is investigated using a small-world neuronal networks in which the pacemaker is stimulated by a square-wave signal. We observe that signal propagation may be significantly enhanced at intermediate temperatures, i.e., temperature favors the propagation of the rhythm of the pacemaker to the whole neuronal network. Furthermore, a rich dynamics is observed, including spiking and bursting activities, as well as full and remote synchronization. We also find that signal propagation crucially depends on the strength of the coupling. Our findings provide new insights on the system dynamics, and improve our understanding of the optimal temperature observed in experiments involving living biological systems.










Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marder, E.: Moving rhythms. Nature 410, 755 (2001)
Koshiya, N., Smith, J.C.: Neuronal pacemaker for breathing visualized in vitro. Nature 400, 360–363 (1999)
Marder, E., Bucher, D.: Central pattern generators and the control of rhythmic movements. Curr. Biol. 11, R986–R996 (2001)
Rabbah, P., Nadim, F.: Distinct synaptic dynamics of heterogeneous pacemaker neurons in an oscillatory network. J. Neurophysiol. 97, 2239–2253 (2007)
Wang, X.J.: Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. J. Neurophysiol. 87, 889–900 (2002)
Gu, H., Ren, W., Lu, Q., Wu, S., Yang, M., Chen, W.: Integer multiple spiking in neuronal pacemakers without external periodic stimulation. Phys. Lett. A 285, 63–68 (2001)
Gu, H.G.: Experimental observation of transition from chaotic bursting to chaotic spiking in a neural pacemaker. Chaos 23, 023126 (2013)
Eisen, J.S., Marder, E.: Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J. Neurophysiol. 48, 1392–1415 (1982)
Nagai, Y., Gonzalez, H., Shrier, A., Glass, L.: Paroxysmal starting and stopping of circulating waves in excitable media. Phys. Rev. Lett. 84, 4248 (2000)
Perc, M., Gosak, M.: Pacemaker-driven stochastic resonance on diffusive and complex networks of bistable oscillators. New J. Phys. 10, 053008 (2008)
Perc, M.: Stochastic resonance on excitable small-world networks via a pacemaker. Phys. Rev. E 76, 066203 (2007)
Kori, H., Mikhailov, A.S.: Entrainment of randomly coupled oscillator networks by a pacemaker. Phys. Rev. Lett. 93, 254101 (2004)
Yao, C.G., Ma, J., Zhiwei He, Z.W., Nakano, T., Qian, Y., Shuai, J.W.: Inhibitory-autapse-enhanced signal transmission in neural networks. Nonliear Dyn. 97, 1425–1437 (2019)
Qin, H., Ma, J., Wang, C., Wu, Y.: Autapse-induced spiral wave in network of neurons under noise. PLoS One 9, e100849 (2014)
Ma, J., Tang, J.: A review for dynamics in neuron and neuronal network. Nonlinear Dyn. 89, 1569–1578 (2017)
Ma, J., Tang, J.: A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045 (2015)
Jia, Y.B., Gu, H.G., Li, Y.Y., Ding, X.L.: Inhibitory autapses enhance coherence resonance of a neuronal network. Commun. Nonlinear Sci. Numer. Simul. 95, 105643 (2021)
Guan, L., Gu, H.G., Zhao, Z.G.: Dynamics of subthreshold and suprathreshold resonance modulated by hyperpolarization-activated cation current in a bursting neuron. Nonlinear Dyn. 104, 577–601 (2021)
Xu, Y., Jia, Y., Wang, H.W., Liu, Y., Wang, P., Zhao, Y.J.: Spiking activities in chain neural network driven by channel noise with field coupling. Nonlinear Dyn. 95, 3237–3247 (2019)
He, Z.W., Yao, C.G.: The effect of oxygen concentration on the coupled neurons: rich spiking patterns and synchronization. Sci. China Technol. Sci. 63, 2339–2348 (2020)
Yao, Z., Wang, C., Zhou, P., Ma, J.: Regulating synchronous patterns in neurons and networks via field coupling. Commun. Nonlinear Sci. Numer. Simul. 95, 105583 (2021)
Lago-Fernandez, L.F., Huerta, R., Corbacho, F., Siguenza, J.A.: Fast response and temporal coherent oscillations in small-world networks. Phys. Rev. Lett. 84, 2758 (2000)
White, J.G., Southgate, E., Thompson, J.N., Brenner, S.: The structure of the nervous system of the nematode caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340 (1986)
Watts, D.J., Strogatz, S.H.: Collective dynamics of small-world networks. Nature 393, 440–442 (1998)
Liu, Y., Xu, Y., Ma, J.: Synchronization and spatial patterns in a light-dependent neural network. Commun. Nonlinear Sci. Numer. Simul. 89, 105297 (2020)
Sun, X.J., Perc, M., Lu, Q.S., Kurths, J.: Effects of correlated Gaussian noise on the mean firing rate and correlations of an electrically coupled neuronal network. Chaos 20, 033116 (2010)
Yao, C.G., Zhan, M., Shuai, J.W., Ma, J., Kurths, J.: Insensitivity of synchronization to network structure in chaotic pendulum systems with time-delay coupling. Chaos 27, 126702 (2017)
Tang, J., Zhang, J., Ma, J., Luo, J.M.: Noise and delay sustained chimera state in small world neuronal network. Sci. China Technol. Sci. 62, 1134–1140 (2019)
Xu, Y., Jia, Y., Ge, M.Y., Lu, L.L., Yang, L.J., Zhan, X.: Effects of ion channel blocks on electrical activity of stochastic Hodgkin-Huxley neural network under electromagnetic induction. Neurocomputing 283, 196–204 (2017)
Ozer, M., Uzuntarla, M., Kayikcioglu, T., Graham, L.J.: Collective temporal coherence for subthreshold signal encoding on a stochastic small-world Hodgkin-Huxley neuronal network. Phys. Lett. A 372, 6498–6503 (2008)
Jun, M., Yang, L.J., Wang, Y., Zhang, C.R.: Spiral wave in small-world networks of Hodgkin-Huxley neurons. Commun. Theor. Phys. 54, 583–588 (2010)
Lv, M., Ma, J., Yao, Y.G., Alzahrani, F.: Synchronization and wave propagation in neuronal network under field coupling. Sci. China Technol. Sci. 62, 448–457 (2019)
Qian, Y., Zhang, C., Wei, Z.G., Liu, F., Yao, C.G., Zheng, Z.G.: The optimal oscillation mode in excitable small-world networks. Europhys. Lett. 131, 38002 (2020)
Bazhenov, M., Timofeev, I., Steriade, M., Sejnowski, T.J.: Potassium model for slow (2–3 Hz) in vivo neocortical paroxysmal oscillations. J. Neurophysiol. 92, 1116–1132 (2004)
Wu, X.X., Shuai, J.W.: Multistability in a neuron model with extracellular potassium dynamics. Phys. Rev. E 85, 061911 (2012)
Wu, X.X., Shuai, J.W.: Effects of extracellular potassium diffusion on electrically coupled neuron networks. Phys. Rev. E 91, 022701 (2015)
Wu, X.X., Yao, C.G., Shuai, J.W.: Enhanced multiple vibrational resonances by Na+ and K+ dynamics in a neuron model. Sci. Rep. 5, 7684 (2015)
Shuai, J.W., Sheng, R., Jung, P.: Entropically modified spiking ability and periodicity in clustered channels. Phys. Rev. E 81, 051913 (2010)
Shuai, J.W., Bikson, M., Hahn, P.J., Lian, J., Durand, D.M.: Ionic mechanisms underlying spontaneous CA1 neuronal firing in Ca\(^{2+}\)-free solution. Biophys. J . 84, 2099–2111 (2003)
Roper, S.N., Obenaus, A., Dudek, F.E.: Osmolality and nonsynaptic epileptiform bursts in rat CA1 and dentate gyrus. Ann. Neurol. 31, 81–85 (1992)
Snow, R.W., Dudek, F.E.: Electrical fields directly contribute to action potential synchronization during convulsant-induced epileptiform bursts. Brain Res. 323, 114–118 (1984)
Wei, Y., Ullah, G., Schiff, S.J.: Unification of neuronal spikes, seizures, and spreading depression. J. Neurosci. 34, 11733–11743 (2014)
Ullah, G., Wei, Y., Dahlem, M.A., Wechselberger, M., Schiff, S.J.: The role of cell volume in the dynamics of seizure, spreading depression, and anoxic depolarization. PLoS Comput. Biol. 14, 1004414 (2015)
Yao, C.G., He, Z.W., Nakano, T., Shuai, J.W.: Spiking patterns of a neuron model to stimulus: Rich dynamics and oxygens role. Chaos 28, 083112 (2018)
Somero, G.N.: Temperature adaptation of enzymes: biological optimization through structure-function compromises. Ann. Rev. Ecol. Syst. 9, 1–29 (1978)
Temperature effects on biological systems: rowbury. Introduction. Sci. Prog. 86, 1–8 (2003)
Schipper, L.A.: On the temperature dependence of enzymecatalyzed rates. Biochemistry 55, 1681–1688 (2016)
Song, X.L., Wang, H.T., Chen, Y., Lai, Y.C.: Emergence of an optimal temperature in action-potential propagation through myelinated axons. Phys. Rev. E 100, 032416 (2019)
Fu, X., Yu, Y.G.: Reliable and efficient processing of sensory information at body temperature by rodent cortical neurons. Nonlinear Dyn. 98, 215–231 (2019)
Yu, Y.G., Shu, Y.S., McCormick, D.A.: Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J. Neurosci. 28, 7260–7272 (2008)
Yu, Y.G., Hill, A.P., McCormick, D.A.: Warm body temperature facilitates energy efficient cortical action potentials. PLoS Comput. Biol. 8, 1002456 (2012)
Tai, C., Wang, J., Roppolo, J.R., Groat, W.C.: Relationship between temperature and stimulation frequency in conduction block of amphibian myelinated axon. J. Comput. Neurosci. 26, 331–338 (2008)
Ding, Q.M., Jia, Y.: Effects of temperature and ion channel blocks on propagation of action potential in myelinated axons. Chaos 31, 053102 (2021)
Yao, C.G., He, Z.W.: Anormal diffusion enhancement of resonant responses for coupled oscillator networks to weak signals. Chaos 30, 083120 (2020)
Peterson, M.E., Daniel, R.M., Danson, M.J., Eisenthal, R.: The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem. J. 402, 331–337 (2007)
Dell, A.I., Pawar, S., Savage, V.M.: Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. U.S.A. 108, 10591–10596 (2011)
Acknowledgements
This work was supported partially by the National Natural Science Foundation of China under Grant Nos. 11675112 and 11805091.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Z., Yao, C., Liu, S. et al. Transmission of pacemaker signal in a small world neuronal networks: temperature effects. Nonlinear Dyn 106, 2547–2557 (2021). https://doi.org/10.1007/s11071-021-06907-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-021-06907-0