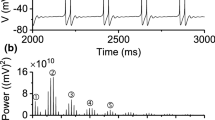
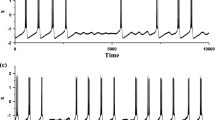
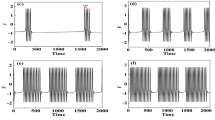
Abstract
Resonance of both subthreshold and suprathreshold membrane potentials has been found in brain or motor neurons involving in the information processing or locomotion control via frequency response, which is mainly related to the hyperpolarization-activated cation current (\(I_{{\text {h}}}\)). In the present paper, the modulations of \(I_{{\text {h}}}\) current on resonance are acquired in a bursting neuron model, which closely match experimental observations. Firstly, the increase of \(I_{{\text {h}}}\) current induces stable node changed to stable focus related to Hopf bifurcation, and to bursting via complex bifurcations including the big homoclinic orbit related to type I excitability. Secondly, the subthreshold resonance can be evoked by small stimulation from focus instead of node, resembling the experimental observation that subthreshold resonance appears at strong (\(I_{{\text {h}}}\)) current. Thirdly, the modulation regularity of \(I_{{\text {h}}}\) current on frequency and amplitude of resonance is simulated, closely matching the experimental observations, which can be well explained with the changing trends of imaginary and real part of eigenvalues of the focus. Last, the suprathreshold bursting is induced by strong stimulation from the stable focus near big homoclinic orbit, resembling that observed in the experiment. The bursting exhibits “suprathreshold resonance” similar to those evoked near Hopf bifurcation corresponding to type II excitability. A novel condition for “suprathreshold resonance” and the underlying codimension-2 bifurcations are acquired. The results present the comprehensive viewpoints on the subthreshold resonance and extend the condition for the “suprathreshold resonance”, which are helpful for deep understanding resonance in the brain or motor neurons with \(I_{{\text {h}}}\) current.
















Similar content being viewed by others
References
Nandakumar, K., Chatterjee, A.: Resonance, parameter estimation, and modal interactions in a strongly nonlinear benchtop oscillator. Nonlinear Dyn. 40(2), 149–167 (2005)
Jin, Y.F., Hu, H.Y.: Principal resonance of a Duffing oscillator with delayed state feedback under narrow-band random parametric excitation. Nonlinear Dyn. 50(1), 213–227 (2006)
Wang, N., Zheng, B., Zheng, H.Y., Yang, B.: When underwater degraded images meet logical stochastic resonance. Nonlinear Dyn. 94(1), 295–305 (2018)
Baysal, V., Saraç, Z., Yilmaz, E.: Chaotic resonance in Hodgkin-Huxley neuron. Nonlinear Dyn. 97(2), 1275–1285 (2019)
Guan, L.N., Gu, H.G., Jia, Y.B.: Multiple coherence resonances evoked from bursting and the underlying bifurcation mechanism. Nonlinear Dyn. 100(4), 3645–3666 (2020)
Liu, C., Zhang, X.Y., Liang, X.M.: Resonance induced by mixed couplings in a three-node motif. Nonlinear Dyn. 102(1), 635–642 (2020)
Lu, L.L., Jia, Y., Ge, M., Xu, Y., Li, A.B.: Inverse stochastic resonance in Hodgkin-Huxley neural system driven by Gaussian and non-Gaussian colored noises. Nonlinear Dyn. 100(1), 877–889 (2020)
Zhang, Y., Xu, Y., Yao, Z., Ma, J.: A feasible neuron for estimating the magnetic field effect. Nonlinear Dyn. (2020). https://doi.org/10.1007/s11071-020-05991-y
Izhikevich, E.M., Desai, N.S., Walcott, E.C., Hoppensteadt, F.C.: Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26(3), 161–167 (2003)
Brunel, N., Hakim, V., Richardson, M.J.: Firing-rate resonance in a generalized integrate-and-fire neuron with subthreshold resonance. Phys. Rev. E 67(5), 051916 (2003)
Richardson, M.J.E., Brunel, N., Hakim, V.: From subthreshold to firing-rate resonance. J. Neurophysiol. 89(5), 2538–2554 (2003)
Remme, M.W., et al.: Subthreshold resonance properties contribute to the efficient coding of auditory spatial cues. Proc. Natl. Acad. Sci. 111(22), E2339-2348 (2014)
Tchumatchenko, T., Clopath, C.: Oscillations emerging from noise-driven steady state in networks with electrical synapses and subthreshold resonance. Nat. Commun. 5, 5512 (2014)
Wang, X.J.: Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 90(3), 1195–1268 (2010)
Tohidi, V., Nadim, F.: Membrane resonance in bursting pacemaker neurons of an oscillatory network is correlated with network frequency. J. Neurosci. 29(20), 6427–6435 (2009)
Fischer, L., Leibold, C., Felmy, F.: Resonance Properties in Auditory Brainstem Neurons. Front. Cell. Neurosci. 12, 8 (2018)
Izhikevich, E.M.: Neural excitability, spiking and bursting. Int. J. Bifurcat. Chaos 10(6), 1171–1266 (2000)
Zhao, Z.G., Gu, H.G.: Transitions between classes of neuronal excitability and bifurcations induced by autapse. Sci. Rep. 7(1), 6760 (2017)
Izhikevich, E.M.: Dynamical systems in neuroscience: the geometry of excitability and bursting. MIT Press, London (2007)
Hutcheon, B., Miura, R.M., Puil, E.: Subthreshold membrane resonance in neocortical neurons. J. Neurophysiol. 76(2), 683–697 (1996)
Vazifehkhah Ghaffari, B., Kouhnavard, M., Aihara, T., Kitajima, T.: Mathematical modeling of subthreshold resonant properties in pyloric dilator neurons. Biomed. Res. Int. 2015, 135787 (2015)
Zhao, Z.G., Li, L., Gu, H.G.: Dynamical mechanism of hyperpolarization-activated non-specific cation current induced resonance and spike-timing precision in a neuronal model. Front. Cell. Neurosci. 12, 62 (2018)
Yu, Y.G., Liu, F., Wang, W.: Frequency sensitivity in Hodgkin-Huxley systems. Biol. Cybern. 84, 227–235 (2001)
Narayanan, R., Johnston, D.: Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56(6), 1061–1075 (2007)
Narayanan, R., Johnston, D.: The h channel mediates location dependence and plasticity of intrinsic phase response in rat hippocampal neurons. J. Neurosci. 28(22), 5846–5860 (2008)
Rathour, R.K., Narayanan, R.: Homeostasis of functional maps in active dendrites emerges in the absence of individual channelostasis. Proc. Natl. Acad. Sci. USA 111(17), E1787-1796 (2014)
Hu, R., Ferguson, K. A., Whiteus, C. B., Meijer, D. H., Araneda, R. C.: Hyperpolarization-activated currents and subthreshold resonance in granule cells of the olfactory bulb. eNeuro 3(5), 1-12 (2016)
Xie, Y., Xu, J.X., Kang, Y.M., Hu, S.J., Duan, Y.B.: Critical amplitude curves for different periodic stimuli and different dynamical mechanisms of excitability. Commun. Nonlinear Sci. Numer. Simul. 10(7), 823–832 (2005)
Nolan, M.F., Dudman, J.T., Dodson, P.D., Santoro, B.: HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J. Neurosci. 27(46), 12440–12451 (2007)
Shay, C.F., Boardman, I.S., James, N.M., Hasselmo, M.E.: Voltage dependence of subthreshold resonance frequency in layer II of medial entorhinal cortex. Hippocampus 22(8), 1733–1749 (2012)
Puil, E., Meiri, H., Yarom, Y.: Resonant behavior and frequency preferences of thalamic neurons. J. Neurophysiol. 71(2), 575–582 (1994)
Ulrich, D.: Subthreshold delta-frequency resonance in thalamic reticular neurons. Eur. J. Neurosci. 40(4), 2600–2607 (2014)
Lampl, I., Yarom, Y.: Subthreshold oscillations and resonant behavior: two manifestations of the same mechanism. Neuroscience 78(2), 325–341 (1997)
Mikiel-Hunter, J., Kotak, V., Rinzel, J.: High-frequency resonance in the gerbil medial superior olive. PLoS Comput. Biol. 12(11), e1005166 (2016)
Howells, J., Bostock, H., Burke, D.: Accommodation to hyperpolarization of human axons assessed in the frequency domain. J. Neurophysiol. 116(2), 322–335 (2016)
Chen, Y.B., Li, X.P., Rotstein, H.G., Nadim, F.: Membrane potential resonance frequency directly influences network frequency through electrical coupling. J. Neurophysiol. 116(4), 1554–1563 (2016)
Lau, T., Zochowski, M.: The resonance frequency shift, pattern formation, and dynamical network reorganization via sub-threshold input. PLoS ONE 6(4), e18983 (2011)
Hutcheon, B., Yarom, Y.: Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23(5), 216–222 (2000)
Bel, A., Rotstein, H.G.: Membrane potential resonance in non-oscillatory neurons interacts with synaptic connectivity to produce network oscillations. J. Comput. Neurosci. 46(2), 169–195 (2019)
Fox, D.M., Tseng, H.A., Smolinski, T.G., Rotstein, H.G., Nadim, F.: Mechanisms of generation of membrane potential resonance in a neuron with multiple resonant ionic currents. PLoS Comput. Biol. 13(6), e1005565 (2017)
Rathour, R.K., Narayanan, R.: Inactivating ion channels augment robustness of subthreshold intrinsic response dynamics to parametric variability in hippocampal model neurons. J. Physiol. 590(22), 5629–5652 (2012)
Rathour, R.K., Malik, R., Narayanan, R.: Transient potassium channels augment degeneracy in hippocampal active dendritic spectral tuning. Sci. Rep. 6, 24678 (2016)
Gutfreund, Y., Yarom, Y., Segev, I.: Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. J. Physiol. 483(3), 621–640 (1995)
Wu, N.P., Hsiao, C.F., Chandler, S.H.: Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. J. Neurosci. 21(11), 3729–3739 (2001)
Kueh, D., Barnett, W.H., Cymbalyuk, G.S., Calabrese, R.L.: Na\({}^+\)/K\({}^+\) pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches. Elife 5, e19322 (2016)
Zhu, L., Selverston, A.I., Ayers, J.: Role of \(I_{{\rm h}}\) in differentiating the dynamics of the gastric and pyloric neurons in the stomatogastric ganglion of the lobster. Homarus americanus. J. Neurophysiol. 115(5), 2434–2445 (2016)
Rich, S., Moradi Chameh, H., Sekulic, V., Valiante, T.A., Skinner, F.K.: Modeling reveals human-rodent differences in h-current kinetics influencing resonance in cortical layer 5 neurons. Cereb. Cortex 00, 1–28 (2020)
Schmidt, S.L., Dorsett, C.R., Iyengar, A.K., Frohlich, F.: Interaction of intrinsic and synaptic currents mediate network resonance driven by layer V pyramidal cells. Cereb. Cortex 27(9), 4396–4410 (2016)
Yeung, M., Dickson, C.T., Treit, D.: Intrahippocampal infusion of the \(\rm {I_h}\) blocker ZD7288 slows evoked theta rhythm and produces anxiolytic-like effects in the elevated plus maze. Hippocampus 23(4), 278–286 (2013)
Stark, E., Eichler, R., Roux, L., Fujisawa, S., Rotstein, H.G., Buzsaki, G.: Inhibition-induced theta resonance in cortical circuits. Neuron 80(5), 1263–1276 (2013)
Brager, D.H., Johnston, D.: Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in \(\rm {I_h}\) in hippocampal CA1 pyramidal neurons. J. Neurosci. 27(51), 13926–13937 (2007)
Tseng, H.A., Nadim, F.: The membrane potential waveform of bursting pacemaker neurons is a predictor of their preferred frequency and the network cycle frequency. J. Neurosci. 30(32), 10809–10819 (2010)
Tseng, H.A., Martinez, D., Nadim, F.: The frequency preference of neurons and synapses in a recurrent oscillatory network. J. Neurosci. 34(38), 12933–12945 (2014)
Dwyer, J., Lee, H., Martell, A., van Drongelen, W.: Resonance in neocortical neurons and networks. Eur. J. Neurosci. 36(12), 3698–3708 (2012)
Hu, H., Vervaeke, K., Storm, J.F.: Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na\({}^+\) current in rat hippocampal pyramidal cells. J. Physiol. 545(3), 783–805 (2002)
Haas, J.S., Dorval, A.D., II., White, J.A.: Contributions of \(I_{{\rm h}}\) to feature selectivity in layer II stellate cells of the entorhinal cortex. J. Comput. Neurosci. 22(2), 161–171 (2007)
Rotstein, H.G., Nadim, F.: Frequency preference in two-dimensional neural models: a linear analysis of the interaction between resonant and amplifying currents. J. Comput. Neurosci. 37(1), 9–28 (2013)
Rotstein, H.G.: Frequency preference response to oscillatory inputs in two-dimensional neural models: a geometric approach to subthreshold amplitude and phase resonance. J. Math. Neurosci. 4, 11 (2014)
Rotstein, H.G.: Subthreshold amplitude and phase resonance in models of quadratic type: nonlinear effects generated by the interplay of resonant and amplifying currents. J. Comput. Neurosci. 38(2), 325–354 (2015)
Barnett, W.H., Cymbalyuk, G.S.: A codimension-2 bifurcation controlling endogenous bursting activity and pulse-triggered responses of a neuron model. PLoS ONE 9(1), e85451 (2014)
Amarillo, Y., Mato, G., Nadal, M.S.: Analysis of the role of the low threshold currents \(\rm {I_{T}}\) and \(\rm {I_{h}}\) in intrinsic delta oscillations of thalamocortical neurons. Front. Comput. Neurosci. 9, 52 (2015)
Xu, K., Maidana, J.P., Caviedes, M., Quero, D., Aguirre, P., Orio, P.: Hyperpolarization-activated current induces period-doubling cascades and chaos in a cold thermoreceptor model. Front. Comput. Neurosci. 11, 12 (2017)
Guan, L.N., Jia, B., Gu, H.G.: A novel threshold across which the negative stimulation evokes action potential near a saddle-node bifurcation in a neuronal model with Ih current. Int. J. Bifurcat. Chaos 29(14), 1950198 (2019)
Wang, J. F., Liu, F., Wang, J. Y., Chen, G., Wang, W.: Frequency characteristics of the input thresholds of stochastic resonant systems. Acta Phys. Sin. 46(12), 2305-2312 (1997) (in chinese)
Parmananda, P., Mena, C.H., Baier, G.: Resonant forcing of a silent Hodgkin-Huxley neuron. Phys. Rev. E 66(4), 047202 (2002)
Wojcik, J., Clewley, R., Shilnikov, A.: Order parameter for bursting polyrhythms in multifunctional central pattern generators. Phys. Rev. E 83(5), 056209 (2011)
van Brederode, J. F., Berger, A. J.: GAD67-GFP\(+\) neurons in the Nucleus of Roller. II. Subthreshold and firing resonance properties. J. Neurophysiol. 105(1), 249-278 (2011)
Dhooge, A., Govaerts, W., Kuznetsov, Y.A.: MATCONT: A MATLAB package for numerical bifurcation analysis of ODEs. Acm Trans. Math. Softw. 29(1), 141–164 (2003)
Ermentrout, B.: Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students. SIAM, Philadelphia (2002)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was sponsored by the National Natural Science Foundation of China (Grant Numbers: 11872276, 11572225, 11802085)
Appendix
Appendix
1.1 The Hodgkin-Huxley (HH) model
The HH model, one of the most important models to describe the neural electrical behaviors, is described as follows:
where
This model contains three major currents: voltage-gated transient \(\mathrm {Na^{+}}\) current, \(I_{{\text {Na}}}=-g_{{\text {Na}}}m^3h(V-E_{{\text {Na}}})\); voltage-gated persistent \(\mathrm {K^{+}}\) current, \(I_{{\text {K}}}=-g_{{\text {K}}}n^4(V-E_{{\text {K}}})\); and ohmic leak current, \(I_{{\text {L}}}=-g_{{\text {L}}}(V-E_{{\text {L}}})\). The variable n is the activation variable for \(\mathrm {K^{+}}\), m and h are the activation variable and inactivation variable for \(\mathrm {Na^{+}}\), respectively. The parameter \(g_{{\text {K}}}\), \(g_{{\text {Na}}}\), and \(g_{{\text {L}}}\) are the maximal conductance of \(I_{{\text {K}}}\), \(I_{{\text {Na}}}\), and \(I_{{\text {L}}}\), respectively, and \(E_{{\text {K}}}\), \(E_{{\text {Na}}}\), and \(E_{{\text {L}}}\) are the reverse potential of the corresponding currents. C is the membrane capacitance, and I is the background current. The parameter values are as follows: C = 1 \(\mu \)F/cm\({}^2\), \(\phi \) = 1, \(g_{{\text {K}}}\) = 36 mS/cm\({}^{2}\), \(g_{{\text {Na}}}\) = 120 mS/cm\({}^{2}\), \(g_{{\text {L}}}\) = 0.3 mS/cm\({}^{2}\), \(E_{{\text {K}}}\) = −77 mV, \(E_{{\text {Na}}}\) = 50 mV, \(E_{{\text {L}}}\) = −54.4 mV. The parameters \(g_{{\text {Na}}}\) and I are taken as bifurcation parameter.
1.2 The one-parameter bifurcation of HH model and comparison to Leech model
For the HH model with \(g_{{\text {Na}}}\) = 120 mS/cm\({}^2\), one-parameter bifurcations with respect to I are shown in Fig. 17. With increasing I, there is a subcritical Hopf bifurcation (subH, I \(\approx \) 9.779273 \(\mu \)A/cm\({}^2\)) through which the stable equilibrium point (focus, red solid curve to the left of the bifurcation point) transforms into unstable equilibrium point (focus, black dashed curve) and a supercritical Hopf bifurcation (supH, I \(\approx \) 154.5263 \(\mu \)A/cm\({}^2\)) via which the unstable equilibrium point transforms to stable equilibrium point (red solid curve to the right of the bifurcation point), as shown in Fig. 17a. An unstable limit cycle (blue dashed curve) bifurcates from the subH point and a stable limit cycle (green solid curve) bifurcates from the supH point coalesce and annihilate each other to form a fold or saddle-node bifurcation (LPC) of limit cycle at \(I \approx 6.264221 \mu \)A/cm\({}^2\). The insert figure in Fig. 17a is the enlargement near the subH and LPC point.
Dynamical behaviors of the HH model when \(g_{{\text {Na}}}\) = 120 mS/cm\({}^2\). a The bifurcations of HH model. The red solid and black dashed curves represent the stable equilibrium and unstable equilibrium respectively. The green solid and blue dashed curves represent the maximum and minimum value of the stable and unstable limit cycle, respectively; The labels subH, supH, and LPC represent the subcritical Hopf, supercritical Hopf, and saddle node of limit cycle bifurcation points respectively. b The subthreshold oscillations evoked from the resting state for I = 2 \(\mu \)A/cm\({}^2\); c Changes of firing frequency with respect to I; d Spike trains of spiking when I = 6.28 \(\mu \)A/cm\({}^2\)
The left stable focus corresponds to the resting state. After a small perturbation to the resting state for I = 2 \(\mu \)A/cm\({}^2\), damping subthreshold oscillations with an intrinsic frequency about 71.84 Hz (the period of subthreshold oscillations is about 13.92 ms) appears, as demonstrated in Fig. 17b.
The stable limit cycle corresponds to spiking behavior. The change of firing frequency with respect to I is shown in Fig. 17c (Insert is the enlargement near LPC point). Near the LPC point, the firing exhibits a non-zero but nearly fixed frequency (\(\sim \)50.26 Hz), implying that HH model is type II excitable system, which is different from the Leech model which shows type I excitability (Fig. 4b of the main text). The firing frequency manifests a slow changing trend with increasing I. For example, the firing frequency is from \(\sim \)50.26 Hz for I = 6.26422 \(\mu \)A/cm\({}^2\) to 70 Hz for I = 10.7196 \(\mu \)A/cm\({}^2\). When I = 6.28 \(\mu \)A/cm\({}^2\), the period of firing is about 19.37 ms and the frequency is about 51.63 Hz. The spike trains for I = 6.28 \(\mu \)A/cm\({}^2\) is shown in Fig. 17d.
a Two-parameter bifurcation and codimension-2 bifurcation of HH model in (I, \(g_{{\text {Na}}}\)) plane. The red dashed, red solid, and blue solid curves represent the subcritical Hopf bifurcation (subH) curve, supercritical Hopf bifurcation (supH) curve, and fold bifurcation (LPC) of limit cycle, respectively. The black triangle represents the codimension-2 bifurcation point, a generalized Hopf (GH) bifurcation point; b Enlargement of a near the codimension-2 bifurcation point
Compared with the one-parameter bifurcation of Leech model shown in Fig. 5 of the main text, two important distinctions can be found. One is that the firing terminates via a big homoclinic orbit bifurcation (BHom) for the Leech model while via a fold bifurcation of limit cycle (LPC) for the HH model, which results in zero frequency for the firing near BHom point of the Leech model and non-zero frequency but a near fixed frequency near LPC point of the HH model. The other is that the LPC and subH is connected by the unstable limit cycle in the HH model, which is different from that no connections appear between the BHom and SubH2 of the Leech model (Fig. 5 in the main text).
1.3 The codimension-2 bifurcation of HH model and comparison to Leech model
No connections between BHom and subH2 further is identified in the two-parameter space in the Leech model (Fig. 8 in the main text), In the present subsection, the relationship between LPC and subH in HH model is identified in a two-parameter space, as demonstrated in Fig. 18. Figure 18b is the enlargement near a codimension-2 bifurcation point labeled with a black triangle in Fig. 18a. In (I, \(g_{{\text {Na}}}\)) plane, the red solid, red dashed, and blue solid curves represent the supercritical Hopf bifurcation (supH), subcritical Hopf (subH) bifurcation, and saddle-node bifurcation of limit cycle (LPC), respectively. The three codimension-1 bifurcation curves intersect to form the codimension-2 bifurcation point labeled with the triangle, which is a generalized Hopf (GH) bifurcation point. Therefore, the LPC and subH in the HH model have relationship, which is that both of them are bifurcated from the GH point. Such a result is different from the Leech model wherein BHom and subH2 have no connections.
1.4 Subthreshold resonance
When I = 2 \(\mu \)A/cm\({}^2\) and \(g_{{\text {Na}}}\) = 120 mS/cm\({}^2\), the HH model neuron is in a resting state corresponding to a stable focus. A Chirp current (\(I_{{\text {Chirp}}}\) = \(0.01sin(2\pi f(t)t)\), where the time-dependent frequency f(t) increases from 0 Hz to 200 Hz in a total duration T of 1000 ms) can evoke subthreshold resonance from the stable focus. The impedance profile exhibits an inverse U shape, which shows that subthreshold resonance appears, as shown in Fig. 19a. The maximal value appears at \(\sim \) 74.0 Hz, which nearly equals the intrinsic frequency of the subthreshold oscillations corresponding to the focus. Such a result shows that focus of both HH model and Leech model exhibits subthreshold resonance, which implies that the subthreshold resonance is mainly determined by the dynamics of focus itself.
1.5 “Suprathreshold resonance”
For the stable focus with I = 2 \(\mu \)A/cm\({}^2\) and \(g_{{\text {Na}}}\) = 120 mS/cm\({}^2\), when a stimulation signal A\(sin(2\pi f_{{\text {sti}}}t\)) is applied, the critical amplitude A to evoke firing exhibits an U-shaped curve with respect to stimulation frequency \(f_{{\text {sti}}}\), which shows that the stimulation signal to induce firing has a preferred frequency and “suprathreshold resonance” appears, as shown in Fig. 19b. Such a result has been reported in Ref [28]. The minimal value appears at \(f_{{\text {sti}}}\) \(\approx \) 64.8 Hz, which is the “suprathreshold resonance” frequency. The \(f_{{\text {sti}}}\) \(\approx \) 64.8 Hz is a value between the firing frequency near LPC point and the subthreshold resonance frequency. The result implies that the “suprathreshold resonance” maybe influenced by the dynamics of the both firing pattern near the focus and the stable focus.
Rights and permissions
About this article
Cite this article
Guan, L., Gu, H. & Zhao, Z. Dynamics of subthreshold and suprathreshold resonance modulated by hyperpolarization-activated cation current in a bursting neuron. Nonlinear Dyn 104, 577–601 (2021). https://doi.org/10.1007/s11071-021-06230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-021-06230-8