Abstract
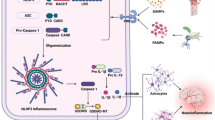
The histone H3 lysine 27 trimethylation (H3K27me3) is one of the most important chromatin modifications, which is associated with injury-activated gene expression in Schwann cells (SCs). However, the alteration of genome-wide H3K27me3 enrichments in the development of neuropathic pain is still unknown. Here, we applied the chromatin immunoprecipitation sequencing (ChIP-seq) approach to identify the alteration of differential enrichments of H3K27me3 in chronic constriction injury (CCI) sciatic nerve of rats and potential molecular mechanisms underlying the development of neuropathic pain. Our results indicated that CCI increased the numbers of SCs displaying H3K27 methyltransferase enhancer of zeste homolog 2 (EZH2) and H3K27me3 in the sciatic nerve. ChIP-seq data showed that CCI significantly changed H3K27me3 enrichments on gene promoters in the sciatic nerve. Bioinformatics analyses exhibited that genes gaining H3K27me3 were mostly associated with regulation of cell proliferation, response to stress and oxidation-reduction process. Genes losing this mark were enriched in neuronal generation, and MAPK, cAMP as well as ERBB signaling pathways. Importantly, IL1A, CCL2, NOS2, S100A8, BDNF, GDNF, ERBB3 and C3 were identified as key genes in neuropathic pain. CCI led to significant upregulation of key genes in the sciatic nerve. EZH2 inhibitor reversed CCI-induced increases of H3K27me3 and key gene protein levels, which were accompanied by relieved mechanical allodynia and thermal hyperalgesia in CCI rats. These results indicate that genes with differential enrichments of H3K27me3 in SCs function in various cellular processes and pathways, and many are linked to neuropathic pain after peripheral nerve injury.




Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- BP:
-
Biological processes
- CCI:
-
Chronic constriction injury
- CCL2:
-
C-C motif chemokine ligand 2
- ChIP-seq:
-
Chromatin immunoprecipitation sequencing
- EZH2:
-
Enhancer of zeste homolog-2
- ERBB3:
-
Receptor tyrosine-protein kinase erbB-3
- GDNF:
-
Glial cell-derived neurotrophic factor
- GO:
-
Gene ontology
- H3K27me3:
-
Histone H3 lysine 27 trimethylation
- IL1A:
-
Interleukin-1α
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- MAG:
-
Myelin-associated glycoprotein
- MWT:
-
Mechanical withdrawal threshold
- NOS2:
-
Nitric oxide synthase 2
- PRC2:
-
Polycomb-repressive complex 2
- SCs:
-
Schwann cells
- TSSs:
-
Transcription start sites
- TWL:
-
Thermal withdrawal latency
References
Wilson ER, Della-Flora Nunes G, Weaver MR, Frick LR, Feltri ML (2021) Schwann cell interactions during the development of the peripheral nervous system. Dev Neurobiol 81:464–489. https://doi.org/10.1002/dneu.22744
Wei Z, Fei Y, Su W, Chen G (2019) Emerging role of Schwann cells in neuropathic pain: receptors, glial mediators and myelination. Front Cell Neurosci 13:116. https://doi.org/10.3389/fncel.2019.00116
Zhu X, Li K, Guo X, Wang J, Xiang Y (2016) Schwann cell proliferation and differentiation that is induced by ferulic acid through MEK1/ERK1/2 signalling promotes peripheral nerve remyelination following crush injury in rats. Exp Ther Med 12:1915–1921. https://doi.org/10.3892/etm.2016.3525
Nocera G, Jacob C (2020) Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci 77:3977–3989. https://doi.org/10.1007/s00018-020-03516-9
Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21:522–527. https://doi.org/10.1016/j.bbi.2006.12.008
An Q, Sun C, Li R, Chen S, Gu X, An S, Wang Z (2021) Calcitonin gene-related peptide regulates spinal microglial activation through the histone H3 lysine 27 trimethylation via enhancer of zeste homolog-2 in rats with neuropathic pain. J Neuroinflammation 18:117. https://doi.org/10.1186/s12974-021-02168-1
Yadav R, Weng HR (2017) EZH2 regulates spinal neuroinflammation in rats with neuropathic pain. Neuroscience 349:106–117. https://doi.org/10.1016/j.neuroscience.2017.02.041
Cai Y, Zhang Y, Loh YP, Tng JQ, Lim MC, Cao Z, Raju A, Lieberman Aiden E, Li S, Manikandan L, Tergaonkar V, Tucker-Kellogg G, Fullwood MJ (2021) H3K27me3-rich genomic regions can function as silencers to repress gene expression via chromatin interactions. Nat Commun 12:719. https://doi.org/10.1038/s41467-021-20940-y
Ma KH, Hung HA, Srinivasan R, Xie H, Orkin SH, Svaren J (2015) Regulation of peripheral nerve myelin maintenance by gene repression through polycomb repressive complex 2. J Neurosci 35:8640–8652. https://doi.org/10.1523/JNEUROSCI.2257-14.2015
Ness JK, Skiles AA, Yap EH, Fajardo EJ, Fiser A, Tapinos N (2016) Nuc-ErbB3 regulates H3K27me3 levels and HMT activity to establish epigenetic repression during peripheral myelination. Glia 64:977–992. https://doi.org/10.1002/glia.22977
Ma KH, Hung HA, Svaren J (2016) Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J Neurosci 36:9135–9147. https://doi.org/10.1523/JNEUROSCI.1370-16.2016
Hou Z, Chen J, Yang H, Hu X, Yang F (2021) PIAS1 alleviates diabetic peripheral neuropathy through SUMOlation of PPAR-γ and mir-124-induced downregulation of EZH2/STAT3. Cell Death Discov 7:372. https://doi.org/10.1038/s41420-021-00765-w
Kidder BL, Hu G, Zhao K (2011) ChIP-Seq: technical considerations for obtaining high-quality data. Nat Immunol 12:918–922. https://doi.org/10.1038/ni.2117
Austin PJ, Wu A, Moalem-Taylor G (2012) Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J Vis Exp 61:3393. https://doi.org/10.3791/3393
Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih IeM, Conejo-Garcia JR, Speicher DW, Zhang R (2015) Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 21:231–238. https://doi.org/10.1038/nm.3799
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A 3rd, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian X, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, Creasy CL (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492(7427):108–112. https://doi.org/10.1038/nature11606
Kobayashi M, Ishibashi S, Tomimitsu H, Yokota T, Mizusawa H (2012) Proliferating immature Schwann cells contribute to nerve regeneration after ischemic peripheral nerve injury. J Neuropathol Exp Neurol 71:511–519. https://doi.org/10.1097/NEN.0b013e318257fe7b
Lee TI, Johnstone SE, Young RA (2006) Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat Protoc 1:729–48. https://doi.org/10.1038/nprot.2006.98
Kelly TK, Liu Y, Lay FD, Liang G, Berman BP, Jones PA (2012) Genome-wide mapping of nucleosome positioning and DNA methylation within individual DNA molecules. Genome Res 22:2497–2506. https://doi.org/10.1101/gr.143008.112
Souroullas GP, Jeck WR, Parker JS, Simon JM, Liu JY, Paulk J, Xiong J, Clark KS, Fedoriw Y, Qi J, Burd CE, Bradner JE, Sharpless NE (2016) An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation. Nat Med 22:632–640. https://doi.org/10.1038/nm.4092
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Creasy CL (2012) EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492(7427):108–112. https://doi.org/10.1038/nature11606
Heinen A, Tzekova N, Graffmann N, Torres KJ, Uhrberg M, Hartung HP, Küry P (2012) Histone methyltransferase enhancer of zeste homolog 2 regulates Schwann cell differentiation. Glia 60:1696–1708. https://doi.org/10.1002/glia.22388
Duman M, Martinez-Moreno M, Jacob C, Tapinos N (2020) Functions of histone modifications and histone modifiers in Schwann cells. Glia 68:1584–1595. https://doi.org/10.1002/glia.23795
Gonçalves NP, Teixeira-Coelho M, Saraiva MJ (2014) The inflammatory response to sciatic nerve injury in a familial amyloidotic polyneuropathy mouse model. Exp Neurol 257:76–87. https://doi.org/10.1016/j.expneurol.2014.04.030
Sobeh M, Mahmoud MF, Rezq S, Alsemeh AE, Sabry OM, Mostafa I, Abdelfattah MAO, El-Allem KA, El-Shazly AM, Yasri A, Wink M (2019) Salix tetrasperma Roxb extract alleviates neuropathic pain in rats via modulation of the NF-κB/TNF-α/NOX/iNOS pathway. Antioxidants (Basel) 8:482. https://doi.org/10.3390/antiox8100482
Green-Fulgham SM, Harland ME, Ball JB, Li J, Lacagnina MJ, D’Angelo H, Dreher RA, Willcox KF, Lorca SA, Kwilasz AJ, Maier SF, Watkins LR, Grace PM (2022) Preconditioning by voluntary wheel running attenuates later neuropathic pain via Nrf2 antioxidant signaling in rats. Pain 163:1939–1951. https://doi.org/10.1097/j.pain.0000000000002589
Marinelli S, Nazio F, Tinari A, Ciarlo L, D’Amelio M, Pieroni L, Vacca V, Urbani A, Cecconi F, Malorni W, Pavone F (2014) Schwann cell autophagy counteracts the onset and chronification of neuropathic pain. Pain 155:93–107. https://doi.org/10.1016/j.pain.2013.09.013
Brosius Lutz A, Lucas TA, Carson GA, Caneda C, Zhou L, Barres BA, Buckwalter MS, Sloan SA (2022) An RNA-sequencing transcriptome of the rodent Schwann cell response to peripheral nerve injury. J Neuroinflam 19:105. https://doi.org/10.1186/s12974-022-02462-6
Sugimoto K, Yasujima M, Yagihashi S (2008) Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des 14:953–961. https://doi.org/10.2174/138161208784139774
Cao Y, Wang Q, Zhou Z, Wang Y, Liu Y, Ji Y, Liu F (2012) Changes of peroxisome proliferator-activated receptor-γ on crushed rat sciatic nerves and differentiated primary Schwann cells. J Mol Neurosci 47:380–388. https://doi.org/10.1007/s12031-011-9662-8
Yi D, Wang K, Zhu B, Li S, Liu X (2021) Identification of neuropathic pain-associated genes and pathways via random walk with restart algorithm. J Neurosurg Sci 65:414–420. https://doi.org/10.23736/S0390-5616.20.04920-6
Bacallao K, Monje PV (2015) Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS ONE 10:e0116948. https://doi.org/10.1371/journal.pone.0116948
Liu L, He L, Yin C, Huang R, Shen W, Ge H, Sun M, Li S, Gao Y, Xiong W (2020) Effects of palmatine on BDNF/TrkB-mediated trigeminal neuralgia. Sci Rep 10:4998. https://doi.org/10.1038/s41598-020-61969-1
Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G (2006) Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci 26:3079–3086. https://doi.org/10.1523/JNEUROSCI.3785-05.2006
Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno DM, Ribeiro E, Peña CJ, Walker D, Bagot RC, Cahill ME, Anderson SA, Labonté B, Hodes GE, Browne H, Chadwick B, Robison AJ, Vialou VF, Dias C, Lorsch Z, Mouzon E, Lobo MK, Dietz DM, Russo SJ, Neve RL, Hurd YL, Nestler EJ (2015) Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci 18:415–422. https://doi.org/10.1038/nn.3932
Zhao T, Wu D, Du J, Liu G, Ji G, Wang Z, Peng F, Man L, Zhou W, Hao A (2022) Folic acid attenuates glial activation in neonatal mice and improves adult mood disorders through epigenetic regulation. Front Pharmacol 13:818423. https://doi.org/10.3389/fphar.2022.818423
Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF (2000) Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci 12:4171–4180
Fang X, Zhang C, Yu Z, Li W, Huang Z, Zhang W (2019) GDNF pretreatment overcomes Schwann cell phenotype mismatch to promote motor axon regeneration via sensory graft. Exp Neurol 318:258–266. https://doi.org/10.1016/j.expneurol.2019.05.011
Bogen O, Joseph EK, Chen X, Levine JD (2008) GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci 28:12–19. https://doi.org/10.1111/j.1460-9568.2008.06308.x
Ma F, Zhang L, Westlund KN (2012) Trigeminal nerve injury ErbB3/ErbB2 promotes mechanical hypersensitivity. Anesthesiology 117:381–388. https://doi.org/10.1097/ALN.0b013e3182604b2b
Fritzinger DC, Benjamin DE (2016) The complement system in neuropathic and postoperative pain. Open Pain J 9:26–37. https://doi.org/10.2174/1876386301609010026
Shamash S, Reichert F, Rotshenker S (2002) The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 22:3052–3060. https://doi.org/10.1523/JNEUROSCI.22-08-03052.2002
Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C (2007) Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 149:706–714. https://doi.org/10.1016/j.neuroscience.2007.08.014
Levy D, Höke A, Zochodne DW (1999) Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci Lett 260:207–209. https://doi.org/10.1016/s0304-3940(98)00982-3
Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, Baranovskaya S, Strongin AY, Shubayev VI (2015) The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem 290:11771–11784. https://doi.org/10.1074/jbc.M114.622316
Luo Y, Fang Y, Kang R, Lenahan C, Gamdzyk M, Zhang Z, Okada T, Tang J, Chen S, Zhang JH (2020) Inhibition of EZH2 (enhancer of zeste homolog 2) attenuates neuroinflammation via H3k27me3/SOCS3/TRAF6/NF-κB (trimethylation of histone 3 lysine 27/suppressor of cytokine signaling 3/tumor necrosis factor receptor family 6/nuclear factor-κB) in a rat model of subarachnoid hemorrhage. Stroke 51:3320–3331. https://doi.org/10.1161/STROKEAHA.120.029951
Zhang X, Wang Y, Yuan J, Li N, Pei S, Xu J, Luo X, Mao C, Liu J, Yu T, Gan S, Zheng Q, Liang Y, Guo W, Qiu J, Constantin G, Jin J, Qin J, Xiao Y (2018) Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J Exp Med 215:1365–1382. https://doi.org/10.1084/jem.20171417
Wang P, Tian H, Zhang Z, Wang Z (2021) EZH2 regulates lipopolysaccharide-induced periodontal ligament stem cell proliferation and osteogenesis through TLR4/MyD88/NF-κB pathway. Stem Cells Int 2021:7625134. https://doi.org/10.1155/2021/7625134
Young MD, Willson TA, Wakefield MJ, Trounson E, Hilton DJ, Blewitt ME, Oshlack A, Majewski IJ (2011) ChIP-seq analysis reveals distinct H3K27me3 profiles that correlate with transcriptional activity. Nucleic Acids Res 39:7415–7427. https://doi.org/10.1093/nar/gkr416
Hui T, Zhao AP, Yang Y, Ye J, Wang L (2018) EZH2 regulates dental pulp inflammation by direct effect on inflammatory factors. Arch Oral Biol 85:16–22. https://doi.org/10.1016/j.archoralbio.2017.10.004
Funding
The study was supported by National Natural Science Foundation of China (No. 3187125; No. 81371234) and Natural Science Foundation of Shandong Province, China (ZR2019MH027).
Author information
Authors and Affiliations
Contributions
SC and XG performed experiments, analyzed data, and wrote the manuscript. SA analyzed and interpreted data, and wrote the manuscript. RL conducted parts of the animal surgery and performed the experiments. ZW provided advice in the design of the study and in interpreting the data and revising the manuscript. All authors have read and approved the final version of the manuscript. The key data are included in figures, tables, and additional files. The full datasets that were analysed are available from the corresponding author on reasonable request. ChIP-seq experiments were performed by KangChen Bio-tech, Shanghai, China.
Corresponding authors
Ethics declarations
Competing Interests
No conflict of interest are declared for any authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Gu, X., Li, R. et al. Genome-wide Analysis of Histone H3 Lysine 27 Trimethylation Profiles in Sciatic Nerve of Chronic Constriction Injury Rats. Neurochem Res 48, 1945–1957 (2023). https://doi.org/10.1007/s11064-023-03879-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03879-y