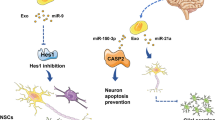
Abstract
Postoperative neurocognitive disorder (PND) is a disease that frequently develops in older patients during the perioperative period. It seriously affects the quality of life of the affected patients. Despite advancements in understanding PND, this disorder’s mechanisms remain unclear, including pathophysiological processes such as central synaptic plasticity and function, neuroinflammation, excitotoxicity, and neurotrophic support. Growing evidence suggests that microenvironmental changes are major factors for PND induction in older individuals. Exosomes are carriers for transporting different bioactive molecules between nerve cells in the microenvironment and maintaining intercellular communication and tissue homeostasis. Studies have shown that exosomes and microRNAs (miRNAs) are involved in various physiological and pathological processes, including neural processes related to PND, such as neurogenesis and cell death, neuroprotection, and neurotrophy. This article reviews the effects of exosomes and miRNAs on the brain microenvironment in PND and has important implications to improve PND diagnosis, as well as to develop targeted therapy of this disorder.




Similar content being viewed by others
Data Availability
No datasets were generated during and/or analysed during the current study, therefore, no data is publicly available.
References
Evered L, Silbert B, Knopman DS et al (2018) Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018[J]. Anesthesiology 129(5):872–879
Shi J, Zou X, Jiang K et al (2020) SIRT1 mediates improvement of cardiac surgery-induced postoperative cognitive dysfunction via the TLR4/NF-κB pathway[J]. world J Biol psychiatry: official J World Federation Soc Biol Psychiatry 21(10):757–765
Gong M, Wang G, Li G et al (2020) Dysfunction of inflammation-resolving pathways is associated with postoperative cognitive decline in elderly mice[J]. Behav Brain Res 386:112538
Yin Q, Ji X, Lv R et al (2020) Targetting Exosomes as a New Biomarker and Therapeutic Approach for Alzheimer’s Disease[J]. Clin Interv Aging 15:195–205
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation[J]. J Neuroinflamm 11:68
Su W, Aloi M, Garden G (2016) MicroRNAs mediating CNS inflammation: Small regulators with powerful potential[J]. Brain Behav Immun 52:1–8
Geng YJ, Wu QH, Zhang RQ (2017) Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: A randomized controlled trial[J]. J Clin Anesth 38:165–171
Guo HY (2017) Significance of interleukin and matrix metalloproteinase in patients with cognitive dysfunction after single valve replacement[J]. Eur Rev Med Pharmacol Sci 21(13):3129–3133
Liu X, Yu Y, Zhu S (2018) Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies[J]. PLoS ONE 13(4):e0195659
Hovens IB, Van Leeuwen BL, Nyakas C et al (2015) Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats[J]. Neurobiol Learn Mem 118:74–79
Hovens IB, Schoemaker RG, Van Der Zee EA et al (2012) Thinking through postoperative cognitive dysfunction: How to bridge the gap between clinical and pre-clinical perspectives[J]. Brain Behav Immun 26(7):1169–1179
Lin X, Chen Y, Zhang P et al (2020) The potential mechanism of postoperative cognitive dysfunction in older people[J]. Exp Gerontol 130:110791
Saxena S, Maze M (2018) Impact on the brain of the inflammatory response to surgery[J]. Presse medicale (Paris, France: 1983), 47: e73-e81
Schaefer ST, Koenigsperger S, Olotu C et al (2019) Biomarkers and postoperative cognitive function: could it be that easy?[J]. Curr Opin Anaesthesiol 32(1):92–100
Yang QQ, Zhou JW (2019) Neuroinflammation in the central nervous system: Symphony of glial cells[J]. Glia 67(6):1017–1035
Xu J, Dong H, Qian Q et al (2017) Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation[J]. Behav Brain Res 332:145–153
Metcalfe M, Figueiredo-Pereira M (2010) Relationship between tau pathology and neuroinflammation in Alzheimer’s disease[J]. Mt Sinai J Med 77(1):50–58
Saman S, Lee N, Inoyo I et al (2014) Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease[J].Journal of Alzheimer’s disease: JAD, :S47-70
Wu D, Gao D, Yu H et al (2021) Medial septum tau accumulation induces spatial memory deficit via disrupting medial septum-hippocampus cholinergic pathway[J]. Clin translational Med 11(6):e428
Jin M, Shepardson N, Yang T et al (2011) Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration[J]. Proc Natl Acad Sci U S A 108(14):5819–5824
Yuyama K, Sun H, Sakai S et al (2014) Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice[J]. J Biol Chem 289(35):24488–24498
Latta C, Sudduth T, Weekman E et al (2015) Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice[J]. J Neuroinflamm 12:41
Abrahamov D, Levran O, Naparstek S et al (2017) Blood-Brain Barrier Disruption After Cardiopulmonary Bypass: Diagnosis and Correlation to Cognition[J]. Ann Thorac Surg 104(1):161–169
Lim A, Krajina K, Marsland AL (2013) Peripheral inflammation and cognitive aging[J]. Mod Trends Pharmacopsychiatry 28:175–187
Li D, Chen M, Meng T et al (2020) Hippocampal microglial activation triggers a neurotoxic-specific astrocyte response and mediates etomidate-induced long-term synaptic inhibition[J]. J Neuroinflamm 17(1):109
Liu Y, Yin Y (2018) Emerging Roles of Immune Cells in Postoperative Cognitive Dysfunction[J]. Mediators Inflamm, 2018: 6215350
Li N, Zhang X, Dong H et al (2017) Bidirectional relationship of mast cells-neurovascular unit communication in neuroinflammation and its involvement in POCD[J]. Behav Brain Res 322:60–69
Safavynia SA, Goldstein PA (2019) The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment[J]. Frontiers in Psychiatry, p 9
Qiu L, Pan W, Luo D et al (2020) Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca/calpain might contribute to postoperative cognitive dysfunction in aging mice[J]. J Neuroinflamm 17(1):23
Kotekar N, Shenkar A, Nagaraj R (2018) Postoperative cognitive dysfunction - current preventive strategies[J]. Clin Interv Aging 13:2267–2273
Biederer T, Kaeser PS, Blanpied TA (2017) Transcell Nanoalignment Synaptic Function[J] Neuron 96(3):680–696
Forner S, Baglietto-Vargas D, Martini A et al (2017) Synaptic Impairment in Alzheimer’s Disease: A Dysregulated Symphony[J]. Trends Neurosci 40(6):347–357
Tetruashvily M, Mcdonald M, Frietze K et al (2016) MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction[J]. Brain Behav Immun 56:197–208
Tan X, Qiu L, Sun J (2021) Research Progress on the Role of Inflammatory Mechanisms in the Development of Postoperative Cognitive Dysfunction[J]. BioMed research international, 2021: 3883204
Yu Y, Jans D, Winblad B et al (2018) Neuronal Aβ42 is enriched in small vesicles at the presynaptic side of synapses[J]. Life Sci alliance 1(3):e201800028
Doens D, Fernández P (2014) Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis[J]. J Neuroinflamm 11:48
Wang G, Dinkins M, He Q et al (2012) Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD)[J]. J Biol Chem 287(25):21384–21395
Lukiw W, Pogue A (2020) Vesicular Transport of Encapsulated microRNA between Glial and Neuronal Cells[J].International journal of molecular sciences, 21(14)
Hoover B, Reed M, Su J et al (2010) Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration[J]. Neuron 68(6):1067–1081
Fang EF, Hou Y, Palikaras K et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease[J]. Nat Neurosci 22(3):401–412
Hou J, Xiao C (2019) Effect of propofol and sevoflurane anesthesia on postoperative cognitive function and levels of Aβ-42 and Tau in patients undergoing hepatectomy[J]. Eur Rev Med Pharmacol Sci 23(2):849–856
Zhang R, Gao Y, Li Y et al (2022) Nrf2 improves hippocampal synaptic plasticity, learning and memory through the circ-Vps41/miR-26a-5p/CaMKIV regulatory network[J].Exp Neurol, :113998
Deng M, Zhang Q, Wu Z et al (2020) Mossy cell synaptic dysfunction causes memory imprecision via miR-128 inhibition of STIM2 in Alzheimer’s disease mouse model[J]. Aging Cell 19(5):e13144
Chen D, Hu S, Wu Z et al (2018) The Role of MiR-132 in Regulating Neural Stem Cell Proliferation, Differentiation and Neuronal Maturation[J]. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 47:2319–23306
Walgrave H, Balusu S, Snoeck S et al (2021) Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease[J]. Cell Stem Cell 28(10):1805–1821e8
Mannironi C, Biundo A, Rajendran S et al (2018) miR-135a Regulates Synaptic Transmission and Anxiety-Like Behavior in Amygdala[J]. Mol Neurobiol 55(4):3301–3315
He E, Lozano M, Stringer S et al (2018) MIR137 schizophrenia-associated locus controls synaptic function by regulating synaptogenesis, synapse maturation and synaptic transmission[J]. Hum Mol Genet 27(11):1879–1891
Fregeac J, Moriceau S, Poli A et al (2020) Loss of the neurodevelopmental disease-associated gene miR-146a impairs neural progenitor differentiation and causes learning and memory deficits[J]. Mol autism 11(1):22
Zhou L, Zhang J, Tan L et al (2021) Elevated Levels of miR-144-3p Induce Cholinergic Degeneration by Impairing the Maturation of NGF in Alzheimer’s Disease[J]. Front cell Dev biology 9:667412
Tang C, Yang J, Liu Q et al (2019) Up-regulated miR-192-5p expression rescues cognitive impairment and restores neural function in mice with depression via the Fbln2-mediated TGF-β1 signaling pathway[J]. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 33(1):606–618
Kumar S, Morton H, Sawant N et al (2021) MicroRNA-455-3p improves synaptic, cognitive functions and extends lifespan: Relevance to Alzheimer’s disease[J]. Redox Biol 48:102182
Fu Y, Hu X, Zheng C et al (2019) Intrahippocampal miR-342-3p inhibition reduces β-amyloid plaques and ameliorates learning and memory in Alzheimer’s disease[J]. Metab Brain Dis 34(5):1355–1363
Guo J, Cai Y, Ye X et al (2019) MiR-409-5p as a Regulator of Neurite Growth Is Down Regulated in APP/PS1 Murine Model of Alzheimer’s Disease[J]. Front NeuroSci 13:1264
Zheng K, Hu F, Zhou Y et al (2021) miR-135a-5p mediates memory and synaptic impairments via the Rock2/Adducin1 signaling pathway in a mouse model of Alzheimer’s disease[J]. Nature communications, 12(1): 1903
Jiang Y, Zhang Y, Su L (2020) MiR-539-5p Decreases amyloid β-protein production, hyperphosphorylation of Tau and Memory Impairment by Regulating PI3K/Akt/GSK-3β Pathways in APP/PS1 Double Transgenic Mice[J]. Neurotox Res 38(2):524–535
Boscher E, Goupil C, Petry S et al (2020) MicroRNA-138 Overexpression Alters Aβ42 Levels and Behavior in Wildtype Mice[J]. Front NeuroSci 14:591138
Zhu Y, Wang Y, Yao R et al (2017) Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period[J]. J Neuroinflamm 14(1):6
Noll F, Behnke J, Leiting S et al (2017) Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation[J]. PLoS ONE 12(12):e0190002
Burmeister AR, Marriott I (2018) The Interleukin-10 Family of Cytokines and Their Role in the CNS[J]. Front Cell Neurosci 12:458
Peng L, Xu L, Ouyang W (2013) Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis[J]. PLoS ONE 8(11):e79624
Marottoli FM, Katsumata Y, Koster KP et al (2017) Peripheral Inflammation, Apolipoprotein E4, and Amyloid-β Interact to Induce Cognitive and Cerebrovascular Dysfunction[J]. ASN Neuro 9(4):1759091417719201
Danielson M, Wiklund A, Granath F et al (2020) Neuroinflammatory markers associate with cognitive decline after major surgery: Findings of an explorative study[J]. Ann Neurol 87(3):370–382
Dev SI, Moore RC, Soontornniyomkij B et al (2017) Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study[J]. Int J Geriatr Psychiatry 32(3):341–349
Balusu S, Van Wonterghem E, De Rycke R et al (2016) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles[J]. EMBO Mol Med 8(10):1162–1183
Rodríguez-Gómez J, Kavanagh E, Engskog-Vlachos P et al (2020) Microglia: Agents of the CNS Pro-Inflammatory Response[J].Cells, 9(7)
Thériault P, Elali A, Rivest S (2015) The dynamics of monocytes and microglia in Alzheimer’s disease[J], vol 7. Alzheimer’s research & therapy, p 41. 1
Lin F, Shan W, Zheng Y et al (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice[J]. J Neurochem 158(2):328–341
Upadhya R, Zingg W, Shetty S et al (2020) Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders[J]. J controlled release: official J Controlled Release Soc 323:225–239
Luo X, Tai W, Sun L et al (2016) Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain[J].Molecular pain,12
Ali I, Chugh D, Ekdahl C (2015) Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain[J]. Neurobiol Dis 74:194–203
Niraula A, Sheridan J, Godbout J (2017) Microglia Priming with Aging and Stress[J]. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 42(1):318–333
Berger M, Oyeyemi D, Olurinde M et al (2019) The INTUIT Study: Investigating Neuroinflammation Underlying Postoperative Cognitive Dysfunction[J]. J Am Geriatr Soc 67(4):794–798
Zhang X, Wang Y, Dong H et al (2016) Induction of Microglial Activation by Mediators Released from Mast Cells[J]. Cell Physiol Biochem 38(4):1520–1531
Zhu H, Liu W, Fang H (2018) Inflammation caused by peripheral immune cells across into injured mouse blood brain barrier can worsen postoperative cognitive dysfunction induced by isoflurane[J]. BMC Cell Biol 19(1):23
Liu H, Wang M, Xu L et al (2021) Neuroprotective effect of miR-204-5p downregulation against isoflurane-induced learning and memory impairment via targeting EphB2 and inhibiting neuroinflammation[J], vol 40. Human & experimental toxicology, pp 1746–1754. 10
Ridder K, Keller S, Dams M et al (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation[J]. PLoS Biol 12(6):e1001874
Saika R, Sakuma H, Noto D et al (2017) MicroRNA-101a regulates microglial morphology and inflammation[J]. J Neuroinflamm 14(1):109
Parisi C, Napoli G, Amadio S et al (2016) MicroRNA-125b regulates microglia activation and motor neuron death in ALS[J]. Cell Death Differ 23(3):531–541
Xing H, Guo S, Zhang Y et al (2016) Upregulation of microRNA-206 enhances lipopolysaccharide-induced inflammation and release of amyloid-β by targeting insulin-like growth factor 1 in microglia[J]. Mol Med Rep 14(2):1357–1364
Prada I, Gabrielli M, Turola E et al (2018) Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations[J]. Acta Neuropathol 135(4):529–550
Long X, Yao X, Jiang Q et al (2020) Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury[J]. J Neuroinflamm 17(1):89
Shao P (2021) MiR-216a-5p ameliorates learning-memory deficits and neuroinflammatory response of Alzheimer’s disease mice via regulation of HMGB1/NF-κB signaling[J]. Brain Res 1766:147511
Song Y, Hu M, Zhang J et al (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease[J]. EBioMedicine 39:409–421
Kumar A, Bhatia H, De Oliveira A et al (2015) microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia[J]. J Neurochem 135(6):1189–1202
Zhang L, Li Y, Wu X et al (2015) MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4[J]. J Neurochem 132(6):713–723
Song M, Jin J, Lim J et al (2011) TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease[J]. J Neuroinflamm 8:92
Hong P, Jiang M, Li H (2014) Functional requirement of dicer1 and miR-17-5p in reactive astrocyte proliferation after spinal cord injury in the mouse[J]. Glia 62(12):2044–2060
Van Scheppingen J, Iyer A, Prabowo A et al (2016) Expression of microRNAs miR21, miR146a, and miR155 in tuberous sclerosis complex cortical tubers and their regulation in human astrocytes and SEGA-derived cell cultures[J]. Glia 64(6):1066–1082
Tu Z, Li Y, Dai Y et al (2017) MiR-140/BDNF axis regulates normal human astrocyte proliferation and LPS-induced IL-6 and TNF-α secretion[J], vol 91. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, pp 899–905
Zhang K, Wu S, Li Z et al (2017) MicroRNA-211/BDNF axis regulates LPS-induced proliferation of normal human astrocyte through PI3K/AKT pathway[J].Bioscience reports, 37(4)
Van Scheppingen J, Mills J, Zimmer T et al (2018) miR147b: A novel key regulator of interleukin 1 beta-mediated inflammation in human astrocytes[J]. Glia 66(5):1082–1097
Duan R, Wang S, Wei B et al (2021) Angiotensin-(1–7) Analogue AVE0991 Modulates Astrocyte-Mediated Neuroinflammation via lncRNA SNHG14/miR-223-3p/NLRP3 Pathway and Offers Neuroprotection in a Transgenic Mouse Model of Alzheimer’s Disease[J]. J Inflamm Res 14:7007–7019
Sun C, Zhu L, Ma R et al (2019) Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes[J], vol 10. Cell death & disease, p 141. 2
Mandolesi G, De Vito F, Musella A et al (2017) miR-142-3p Is a Key Regulator of IL-1β-Dependent Synaptopathy in Neuroinflammation[J]. J neuroscience: official J Soc Neurosci 37(3):546–561
Xu L, Qiu X, Wang S et al (2019) NMDA Receptor Antagonist MK801 Protects Against 1-Bromopropane-Induced Cognitive Dysfunction[J]. Neurosci Bull 35(2):347–361
Hridi SU, Franssen A, Jiang HR et al (2019) Interleukin-16 inhibits sodium channel function and GluA1 phosphorylation via CD4- and CD9-independent mechanisms to reduce hippocampal neuronal excitability and synaptic activity[J]. Mol Cell Neurosci 95:71–78
Hsieh H, Boehm J, Sato C et al (2006) AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss[J]. Neuron 52(5):831–843
Monteiro-Fernandes D, Silva J, Soares-Cunha C et al (2021) Allosteric modulation of AMPA receptors counteracts Tau-related excitotoxic synaptic signaling and memory deficits in stress- and Aβ-evoked hippocampal pathology[J]. Mol Psychiatry 26(10):5899–5911
Rivero-Segura NA, Coronado-Mares MI, Rincón-Heredia R et al (2019) Prolactin prevents mitochondrial dysfunction induced by glutamate excitotoxicity in hippocampal neurons[J]. Neurosci Lett 701:58–64
Dou Y, Xie J, Tan Y et al (2021) Neurotransmitter-stimulated neuron-derived sEVs have opposite effects on amyloid β-induced neuronal damage[J]. J Nanobiotechnol 19(1):324
Reigada D, Calderón-García A, Soto-Catalán M et al (2019) MicroRNA-135a-5p reduces P2X -dependent rise in intracellular calcium and protects against excitotoxicity[J]. J Neurochem 151(1):116–130
Aw S, Lim I, Tang M et al (2017) A Glio-Protective Role of mir-263a by Tuning Sensitivity to Glutamate[J]. Cell Rep 19(9):1783–1793
Shakespear N, Ogura M, Yamaki J et al (2020) Astrocyte-Derived Exosomal microRNA miR-200a-3p Prevents MPP-Induced Apoptotic Cell Death Through Down-Regulation of MKK4[J]. Neurochem Res 45(5):1020–1033
Mollinari C, Racaniello M, Berry A et al (2015) miR-34a regulates cell proliferation, morphology and function of newborn neurons resulting in improved behavioural outcomes[J], vol 6. Cell death & disease, p e1622
Wang T, Cai Q, Yang W et al (2018) MicroRNA-219 alleviates glutamate-induced neurotoxicity in cultured hippocampal neurons by targeting calmodulin-dependent protein kinase II gamma[J]. Neural regeneration research 13(7):1216–1224
Fu C, Han X, Tong L et al (2021) miR-142 downregulation alleviates the impairment of spatial learning and memory, reduces the level of apoptosis, and upregulates the expression of pCaMKII and BAI3 in the hippocampus of APP/PS1 transgenic mice[J]. Behav Brain Res 414:113485
Alsharafi W, Luo Z, Long X et al (1979) MicroRNA in glutamate receptor-dependent neurological diseases[J]. Clinical science (London, England: 2017, 131(14): 1591–1604
Gu Q, Yu D, Hu Z et al (2015) miR-26a and miR-384-5p are required for LTP maintenance and spine enlargement[J]. Nat Commun 6:6789
Harraz M, Eacker S, Wang X et al (2012) MicroRNA-223 is neuroprotective by targeting glutamate receptors[J]. Proc Natl Acad Sci USA 109(46):18962–18967
Corbel C, Hernandez I, Wu B et al (2015) Developmental attenuation of N-methyl-D-aspartate receptor subunit expression by microRNAs[J]. Neural Dev 10:20
Hu Z, Yu D, Gu Q et al (2014) miR-191 and miR-135 are required for long-lasting spine remodelling associated with synaptic long-term depression[J]. Nat Commun 5:3263
Hu Z, Zhao J, Hu T et al (2015) miR-501-3p mediates the activity-dependent regulation of the expression of AMPA receptor subunit GluA1[J]. J Cell Biol 208(7):949–959
Ryan B, Logan B, Abraham W et al (2017) MicroRNAs, miR-23a-3p and miR-151-3p, Are Regulated in Dentate Gyrus Neuropil following Induction of Long-Term Potentiation In Vivo[J]. PLoS ONE 12(1):e0170407
Lu S, Fu C, Liang L et al (2021) miR– regulates cognitive functions in the hippocampus through complement component 3-dependent modulation of synaptic vesicle release[J].Proceedings of the National Academy of Sciences of the United States of America, 118(14)
Mathew R, Tatarakis A, Rudenko A et al (2016) A microRNA negative feedback loop downregulates vesicle transport and inhibits fear memory[J].eLife, 5
Rodriguez-Ortiz C, Prieto G, Martini A et al (2020) miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease[J]. Aging Cell 19(3):e13118
Wang X, Liu D, Huang H et al (2018) A Novel MicroRNA-124/PTPN1 Signal Pathway Mediates Synaptic and Memory Deficits in Alzheimer’s Disease[J]. Biol Psychiatry 83(5):395–405
Silva M, Rodrigues B, Fernandes J et al (2019) MicroRNA-186-5p controls GluA2 surface expression and synaptic scaling in hippocampal neurons[J]. Proc Natl Acad Sci USA 116(12):5727–5736
Xu Y, Chen P, Wang X et al (2018) miR-34a deficiency in APP/PS1 mice promotes cognitive function by increasing synaptic plasticity via AMPA and NMDA receptors[J]. Neurosci Lett 670:94–104
Morquette B, Juźwik C, Drake S et al (2019) MicroRNA-223 protects neurons from degeneration in experimental autoimmune encephalomyelitis[J]. Brain 142(10):2979–2995
Panja D, Li Y, Ward M et al (2021) miR-936 is Increased in Schizophrenia and Inhibits Neural Development and AMPA Receptor-Mediated Synaptic Transmission[J]. Schizophr Bull 47(6):1795–1805
Xiao G, Chen Q, Zhang X (2021) MicroRNA-455-5p/CPEB1 pathway mediates Aβ-related learning and memory deficits in a mouse model of Alzheimer’s disease[J]. Brain Res Bull 177:282–294
Meng X, Zhong J, Zeng C et al (2021) MiR-30a-5p Regulates GLT-1 Function via a PKCα-Mediated Ubiquitin Degradation Pathway in a Mouse Model of Parkinson’s Disease[J]. ACS Chem Neurosci 12(9):1578–1592
Batool S, Raza H, Zaidi J et al (2019) Synapse formation: from cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders[J]. J Neurophysiol 121(4):1381–1397
Erickson KI, Miller DL, Roecklein KA (2012) The aging hippocampus: interactions between exercise, depression, and BDNF[J]. Neuroscientist 18(1):82–97
Numakawa T, Suzuki S, Kumamaru E et al (2010) BDNF function and intracellular signaling in neurons[J]. Histol Histopathol 25(2):237–258
Kowiański P, Lietzau G, Czuba E et al (2018) BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity[J]. Cell Mol Neurobiol 38(3):579–593
Guan W, Xu D, Ji C et al (2021) Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF[J]. Pharmacol Res 174:105932
Huan Z, Mei Z, Na H et al (2021) lncRNA MIR155HG Alleviates Depression-Like Behaviors in Mice by Regulating the miR-155/BDNF Axis[J]. Neurochem Res 46(4):935–944
Mohammadipoor-Ghasemabad L, Sangtarash M, Sheibani V et al (2019) Hippocampal microRNA-191a-5p Regulates BDNF Expression and Shows Correlation with Cognitive Impairment Induced by Paradoxical Sleep Deprivation[J]. Neuroscience 414:49–59
Zhan Y, Han J, Xia J et al (2021) Berberine Suppresses Mice Depression Behaviors and Promotes Hippocampal Neurons Growth Through Regulating the miR-34b-5p/miR-470-5p/BDNF Axis[J]. Neuropsychiatr Dis Treat 17:613–626
Zheng P, Bin H, Chen W (2019) Inhibition of microRNA-103a inhibits the activation of astrocytes in hippocampus tissues and improves the pathological injury of neurons of epilepsy rats by regulating BDNF[J]. Cancer Cell Int 19(1):109
Wang Y, Zhao L, Kan B et al (2018) miR-22 exerts anti-alzheimic effects via the regulation of apoptosis of hippocampal neurons[J]. Cellular and molecular biology. France) 64(15):84–89(Noisy-le-Grand
Ding Y, Luan W, Shen X et al (2022) LncRNA BDNF-AS as ceRNA regulates the miR-9-5p/BACE1 pathway affecting neurotoxicity in Alzheimer’s disease[J]. Arch Gerontol Geriatr 99:104614
Baby N, Alagappan N, Dheen S et al (2020) MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Aβ(1–42)-induced model of Alzheimer’s disease[J]. Aging Cell 19(1):e13046
Gupta N, Jadhav S, Tan K et al (2020) miR-142-3p Regulates BDNF Expression in Activated Rodent Microglia Through Its Target CAMK2A[J]. Front Cell Neurosci 14:132
Pan S, Feng W, Li Y et al (2021) The microRNA-195 - BDNF pathway and cognitive deficits in schizophrenia patients with minimal antipsychotic medication exposure[J]. Translational psychiatry 11(1):117
Wei C, Sun Y, Wang J et al (2021) LncRNA NONMMUT055714 acts as the sponge of microRNA-7684-5p to protect against postoperative cognitive dysfunction[J]. Aging 13(9):12552–12564
Xu L, Xu Q, Xu F et al (2020) MicroRNA-325-3p prevents sevoflurane-induced learning and memory impairment by inhibiting Nupr1 and C/EBPβ/IGFBP5 signaling in rats[J]. Aging 12(6):5209–5220
Liu Q, Hou A, Zhang Y et al (2019) MiR-190a potentially ameliorates postoperative cognitive dysfunction by regulating Tiam1[J]. BMC Genomics 20(1):670
Zhang N, Ye W, Wang T et al (2020) Up-regulation of miR-106a targets LIMK1 and contributes to cognitive impairment induced by isoflurane anesthesia in mice[J], vol 42. Genes & genomics, pp 405–412. 4
Yu Y, Zhang W, Zhu D et al (2021) LncRNA Rian ameliorates sevoflurane anesthesia-induced cognitive dysfunction through regulation of miR-143-3p/LIMK1 axis[J]. Hum Cell 34(3):808–818
Zhang Y, Liu J, Xie C et al (2021) Overexpression of miR-133b protects against isoflurane-induced learning and memory impairment[J]. Experimental and therapeutic medicine 22(5):1207
Chen L, Dong R, Lu Y et al (2019) MicroRNA-146a protects against cognitive decline induced by surgical trauma by suppressing hippocampal neuroinflammation in mice[J]. Brain Behav Immun 78:188–201
Lu Y, Xu X, Dong R et al (2019) MicroRNA-181b-5p attenuates early postoperative cognitive dysfunction by suppressing hippocampal neuroinflammation in mice[J]. Cytokine 120:41–53
Wang L, Liu W, Zhang Y et al (2020) Dexmedetomidine had neuroprotective effects on hippocampal neuronal cells via targeting lncRNA SHNG16 mediated microRNA-10b-5p/BDNF axis[J]. Mol Cell Biochem 469:41–51
Wang Y, Zhang Y, Cai D (2021) Dexmedetomidine Ameliorates Postoperative Cognitive Dysfunction via the MicroRNA-381-Mediated EGR1/p53 Axis[J]. Mol Neurobiol 58(10):5052–5066
Szwed K, Szwed M, Kozakiewicz M et al (2021) Circulating MicroRNAs and Novel Proteins as Potential Biomarkers of Neurological Complications after Heart Bypass Surgery[J].Journal of clinical medicine, 10(14)
Kanninen K, Bister N, Koistinaho J et al (2016) Exosomes as new diagnostic tools in CNS diseases[J]. Biochim Biophys Acta 1862(3):403–410
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception and design. Paper collection and analysis were performed by Qiao-mei Huang and Ying-ying Zhou. The first draft of the manuscript was written by Qiao-mei Huang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
As this was a review article, no informed consent was required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Qm., Zhou, Yy., He, Hf. et al. Research Progress on Exosomes and MicroRNAs in the Microenvironment of Postoperative Neurocognitive Disorders. Neurochem Res 47, 3583–3597 (2022). https://doi.org/10.1007/s11064-022-03785-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03785-9