Abstract
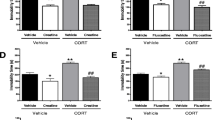
Brain derived neurotrophic factor (BDNF) is one of the most abundant neurotrophic factors, and its deficits are involved in the pathogenesis of major depressive disorders (MDD). Loureirin C (Lou C) is a compound derived from red resin extracted from the stems of Chinese dragon's blood. Xanthoceraside (Xan) is a triterpenoid saponin extracted from the husks of Xanthoceras sorbifolia Bunge. These compounds have neuroprotective effects through upregulation of BDNF. The present study aimed to evaluate whether Lou C and Xan attenuate abnormal behaviors induced by chronic corticosterone (CORT) administration. CORT was administered subcutaneously to mice for 3 weeks, and Lou C and Xan, dispensed orally once a day during the last 2 weeks of CORT administration. Chronic CORT administration induced abnormal behaviors such as prolonged starting latency in the open field test, decreased social interaction time in the social interaction test and prolonged latency to eat in the novelty suppressed feeding test. Chronic CORT administration decreased the expression levels of BDNF and the phosphorylation of protein kinase B (Akt), mammalian target of rapamycin (mTOR), and the cAMP response element binding protein (CREB) in the prefrontal cortex. Lou C and Xan dose-dependently prevented the abnormal behaviors and decreased the expression levels of BDNF and in phosphorylation of AKT, mTOR, and CREB in the prefrontal cortex of CORT mice. These results suggest that Lou C and Xan could be attractive candidates for pharmacotherapy of MDD at least in part, given their propensity to increase BDNF expression and phosphorylation of AKT, mTOR, and CREB.







Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain derived neurotrophic factor
- CORT:
-
Corticosterone
- Lou C:
-
Loureirin C
- MDD:
-
Major depressive disorders
- ERK:
-
Extracellular signal-regulated kinase
- Akt:
-
Protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- CREB:
-
CAMP response element binding protein
- TrkB:
-
Tropomyosin related kinase B
- 7,8-DHF:
-
7,8-Dihydroxyflavone
- HPA:
-
Hypothalamic–pituitary–adrenal
- PI3K:
-
Phosphatidylinositol 3-kinase
- PFC:
-
Prefrontal cortex
- NAc:
-
Nucleus accumbens
- HIP:
-
Hippocampus
- Xan:
-
Xanthoceraside
References
Kessler RC (2012) The costs of depression. Psychiatr Clin North Am 35:1–14
Simon GE (2003) Social and economic burden of mood disorders. Biol Psychiatry 54:208–215
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917
Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD (1990) NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5:501–509
Daskalakis NP, De Kloet ER, Yehuda R, Malaspina D, Kranz TM (2015) Early life stress effects on glucocorticoid-BDNF interplay in the hippocampus. Front Mol Neurosci 8:68
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT (2001) Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50:260–265
Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC (2007) Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry 62:1217–1227
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547
Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23:349–357
Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM (1997) Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 56:131–137
Yoshii A, Constantine-Paton M (2010) Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol 70:304–322
Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel JJ, Becker C (2011) Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci 31:12889–12899
Sun HF, Song MF, Zhang Y, Zhang ZL (2021) Transcriptome profiling reveals candidate flavonoid-related genes during formation of dragon’s blood from Dracaena cochinchinensis (Lour.) S.C.Chen under conditions of wounding stress. J Ethnopharmacol 273:113987
Fan JY, Yi T, Sze-To CM, Zhu L, Peng WL, Zhang YZ, Zhao ZZ, Chen HB (2014) A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine “dragon’s blood.” Molecules 19:10650–10669
Yang L, Ran Y, Quan Z, Wang R, Yang Q, Jia Q, Zhang H, Li Y, Peng Y, Liang J, Wang H, Nakanishi H, Deng Y, Qing H (2019) Pterostilbene, an active component of the dragon’s blood extract, acts as an antidepressant in adult rats. Psychopharmacology 236:1323–1333
Li N, Ma Z, Li M, Xing Y, Hou Y (2014) Natural potential therapeutic agents of neurodegenerative diseases from the traditional herbal medicine Chinese dragon’s blood. J Ethnopharmacol 152:508–521
Qi Y, Zou LB, Wang LH, Jin G, Pan JJ, Chi TY, Ji XF (2013) Xanthoceraside inhibits pro-inflammatory cytokine expression in Abeta25-35/IFN-gamma-stimulated microglia through the TLR2 receptor, MyD88, nuclear factor-kappaB, and mitogen-activated protein kinase signaling pathways. J Pharmacol Sci 122:305–317
Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, Zou LB, Nabeshima T (2012) Xanthoceraside attenuates amyloid beta peptide(2)(5)(-)(3)(5)-induced learning and memory impairments in mice. Psychopharmacology 219:181–190
Yang CY, Ha W, Lin Y, Jiang K, Yang JL, Shi YP (2016) Polyphenols isolated from Xanthoceras sorbifolia husks and their anti-tumor and radical-scavenging activities. Molecules 21:1694
Zang E, Qiu B, Chen N, Li C, Liu Q, Zhang M, Liu Y, Li M (2021) Xanthoceras sorbifolium Bunge: a review on botany, phytochemistry, pharmacology, and applications. Front Pharmacol 12:708549
Li Y, Xu J, Xu P, Song S, Liu P, Chi T, Ji X, Jin G, Qiu S, Hou Y, Zheng C, Wang L, Meng D, Zou L (2016) Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci Lett 629:208–214
Gilman SE, Trinh NH, Smoller JW, Fava M, Murphy JM, Breslau J (2013) Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychol Med 43:303–316
McLaughlin KA, Conron KJ, Koenen KC, Gilman SE (2010) Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med 40:1647–1658
Naughton M, Dinan TG, Scott LV (2014) Corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal axis in psychiatric disease. Handb Clin Neurol 124:69–91
Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126
Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW (2009) Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626
Zhou H, Li X, Gao M (2009) Curcumin protects PC12 cells from corticosterone-induced cytotoxicity: possible involvement of the ERK1/2 pathway. Basic Clin Pharmacol Toxicol 104:236–240
Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM (2013) Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38:872–883
Kim YK, Na KS, Myint AM, Leonard BE (2016) The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry 64:277–284
Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E (2005) Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 53:129–139
Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X (2006) Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 1122:56–64
Huang Z, Zhong XM, Li ZY, Feng CR, Pan AJ, Mao QQ (2011) Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci Lett 493:145–148
Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E (1998) Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res 813:112–120
Chi TY, Wang LH, Qu C, Yang BZ, Ji XF, Wang Y, Okuyama T, Yoshihito O, Zou LB (2009) Protective effects of xanthoceraside on learning and memory impairment induced by Abeta(25–35) in mice. J Asian Nat Prod Res 11:1019–1027
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581:113–120
Mouri A, Sasaki A, Watanabe K, Sogawa C, Kitayama S, Mamiya T, Miyamoto Y, Yamada K, Noda Y, Nabeshima T (2012) MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J Neurosci 32:4562–4580
Mouri A, Lee HJ, Mamiya T, Aoyama Y, Matsumoto Y, Kubota H, Huang WJ, Chiou LC, Nabeshima T (2020) Hispidulin attenuates the social withdrawal in isolated disrupted-in-schizophrenia-1 mutant and chronic phencyclidine-treated mice. Br J Pharmacol 177:3210–3224
Lu Q, Mouri A, Yang Y, Kunisawa K, Teshigawara T, Hirakawa M, Mori Y, Yamamoto Y, Libo Z, Nabeshima T, Saito K (2019) Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav Brain Res 372:112053
Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y, Nitta A, Furukawa H, Nabeshima T (2007) Involvement of a dysfunctional dopamine-D1/N-methyl-d-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol 71:1598–1609
Pereira GC, Roversi K, Trevisan G, Burger ME, Bochi GV (2020) Glucocorticoid and brain-derived neurotrophic factor relationship: a brief investigation into the model of depression by chronic administration of corticosterone. Behav Pharmacol 31:407–412
Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL (2014) proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep 7:796–806
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B (2005) Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci 8:1069–1077
Xue Z, Shui M, Lin X, Sun Y, Liu J, Wei C, Wu A, Li T (2022) Role of BDNF/ProBDNF imbalance in postoperative cognitive dysfunction by modulating synaptic plasticity in aged mice. Front Aging Neurosci 14:780972
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33:67–75
Marsden WN (2013) Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry 43:168–184
Groves JO (2007) Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry 12:1079–1088
Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Swaab DF, Czeh B (2014) Neuropathology of stress. Acta Neuropathol 127:109–135
Fernandez F, Misilmeri MA, Felger JC, Devine DP (2004) Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology 29:59–71
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Blasco-Serra A, Gonzalez-Soler EM, Cervera-Ferri A, Teruel-Marti V, Valverde-Navarro AA (2017) A standardization of the novelty-suppressed feeding test protocol in rats. Neurosci Lett 658:73–78
Chaudhury D, Liu H, Han MH (2015) Neuronal correlates of depression. Cell Mol Life Sci 72:4825–4848
Belleau EL, Treadway MT, Pizzagalli DA (2019) The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry 85:443–453
Duman RS, Voleti B (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Agasse F, Mendez-David I, Christaller W, Carpentier R, Braz BY, David DJ, Saudou F, Humbert S (2020) Chronic corticosterone elevation suppresses adult hippocampal neurogenesis by hyperphosphorylating huntingtin. Cell Rep 32:107865
Gong Q, Yan XJ, Lei F, Wang ML, He LL, Luo YY, Gao HW, Feng YL, Yang SL, Li J, Du LJ (2019) Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: pivotal sites of oxidative phosphorylation. Mol Brain 12:118
Ahima R, Krozowski Z, Harlan R (1991) Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol 313:522–538
Arriza JL, Simerly RB, Swanson LW, Evans RM (1988) The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron 1:887–900
van Eekelen JA, Bohn MC, de Kloet ER (1991) Postnatal ontogeny of mineralocorticoid and glucocorticoid receptor gene expression in regions of the rat tel- and diencephalon. Brain Res Dev Brain Res 61:33–43
Hartmann J, Dedic N, Pohlmann ML, Hausl A, Karst H, Engelhardt C, Westerholz S, Wagner KV, Labermaier C, Hoeijmakers L, Kertokarijo M, Chen A, Joels M, Deussing JM, Schmidt MV (2017) Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry 22:466–475
Reul JM, Collins A, Saliba RS, Mifsud KR, Carter SD, Gutierrez-Mecinas M, Qian X, Linthorst AC (2015) Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress 1:44–59
Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, McCullough KM, Lardenoije R, Joels M, Meijer OC, McCann KE, Dudek SM, Sarabdjitsingh RA, Daskalakis NP, Klengel T, Gassen NC, Schmidt MV, Ressler KJ (2021) Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep 35:109185
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, SDS Team (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Acknowledgements
We thank our lab members for helpful discussions. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (17H04252, 20K07931, and 20K16679), by the Private University Research Branding Project from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), and by Japan Science and Technology Agency (JST) FOREST Program (JPMJFR215H). This work was supported by a grant from the Education and Research Facility of Animal Models for Human Diseases at Fujita Health University, by a research grant from the Smoking Research Foundation, and by the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
YY, QL and AM devised the project and the main conceptual ideas, conducted the experiments, and wrote the manuscript. KK, HK, MH, MH, and YM assisted with the experiments. KS and ZL contributed to the discussion section in the manuscript. TN and AM supervised the study and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Mouri, A., Lu, Q. et al. Loureirin C and Xanthoceraside Prevent Abnormal Behaviors Associated with Downregulation of Brain Derived Neurotrophic Factor and AKT/mTOR/CREB Signaling in the Prefrontal Cortex Induced by Chronic Corticosterone Exposure in Mice. Neurochem Res 47, 2865–2879 (2022). https://doi.org/10.1007/s11064-022-03694-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03694-x