Abstract
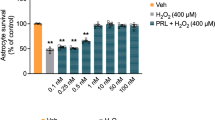
Our previous studies revealed that the expression of stanniocalcin-1 (STC1) in astrocytes increased under hypoxic conditions. However, the role of STC1 in hypoxic astrocytes is not well understood. In this work, we first showed the increased expression of STC1 in astrocyte cell line and astrocytes in the brain tissues of mice after exposure to hypoxia. Then, we found that knockdown of STC1 inhibited cell viability and increased apoptosis. These effects were mediated by decreasing the levels of SIRT3, UCP2, and glycolytic genes and increasing the levels of ROS. Further studies suggested that STC1 silencing promoted oxidative stress and suppressed glycolysis by downregulating AMPKα1. Moreover, HIF-1α knockdown in hypoxic astrocytes led to decreased expression of STC1 and AMPKα1, indicating that the expression of STC1 was regulated by HIF-1α. In conclusion, our study showed that HIF-1α-induced STC1 could protect astrocytes from hypoxic damage by regulating glycolysis and redox homeostasis in an AMPKα1-dependent manner.






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ALDOA:
-
Aldolase A
- AMPK:
-
AMP-Activated Protein Kinase
- ERK:
-
Extracellular Signal-Regulated Protein Kinases
- GDNF:
-
Glial Cell Line-Derived Neurotrophic Factor
- GLUT1:
-
Glucose Transporter 1
- HIF-1α:
-
Hypoxia Inducible Factor-1α
- IHC:
-
Immunohistochemical (IHC)
- LDHA:
-
Lactate Dehydrogenase A
- NRF2:
-
Nuclear Factor E2-Related Factor 2
- PGK1:
-
Phosphoglycerate Kinase 1
- ROS:
-
Reactive Oxygen Species
- SIRT3:
-
Sirtuin 3
- STC1:
-
Stanniocalcin-1
- UCP2:
-
Uncoupling Protein 2
References
Santello M et al (2019) Astrocyte function from information processing to cognition and cognitive impairment. Nat Neurosci 22:154–166. https://doi.org/10.1038/s41593-018-0325-8
Vangeison G, Rempe DA (2009) The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist 15:579–588. https://doi.org/10.1177/1073858409332405
Marina N et al (2016) Astrocytes and brain hypoxia. Adv Exp Med Biol 903:201–207. https://doi.org/10.1007/978-1-4899-7678-9_14
Mitroshina ЕV et al (2019) Intracellular neuroprotective mechanisms in neuron-glial networks mediated by glial cell line-derived neurotrophic factor. Oxid Med Cell Longev 2019:1036907. https://doi.org/10.1155/2019/1036907
Toriuchi K et al (2020) Prolonged astrocyte-derived erythropoietin expression attenuates neuronal damage under hypothermic conditions. J Neuroinflammation 17:141. https://doi.org/10.1186/s12974-020-01831-3
Bolanos JP (2016) Bioenergetics and redox adaptations of astrocytes to neuronal activity. J Neurochem 139 Suppl 2:115–125. https://doi.org/10.1111/jnc.13486
Brekke E et al (2015) Glucose metabolism and astrocyte-neuron interactions in the neonatal brain. Neurochem Int 82:33–41. https://doi.org/10.1016/j.neuint.2015.02.002
Chen K et al (2018) Lactate transport facilitates neurite outgrowth. Biosci Rep 38:BSR20180157. https://doi.org/10.1042/BSR20180157
Gao Y et al (2019) Integrated analysis identified core signal pathways and hypoxic characteristics of human glioblastoma. J Cell Mol Med 23:6228–6237. https://doi.org/10.1111/jcmm.14507
Zhao F et al (2020) Expression, function and clinical application of stanniocalcin-1 in cancer. J Cell Mol Med 24:7686–7696. https://doi.org/10.1111/jcmm.15348
Pan JS et al (2015) Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol 26:364–378. https://doi.org/10.1681/ASN.2013070703
Liu D et al (2016) Stanniocalcin-1 protects a mouse model from renal ischemia-reperfusion injury by affecting ROS-mediated multiple signaling pathways. Int J Mol Sci 17:1051. https://doi.org/10.3390/ijms17071051
Huang L et al (2012) Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 82:867–877. https://doi.org/10.1038/ki.2012.223
Zhao F et al (2020) Stanniocalcin-1 alleviates contrast-induced acute kidney injury by regulating mitochondrial quality control via the Nrf2 pathway. Oxid Med Cell Longev 2020:1898213–1898213. https://doi.org/10.1155/2020/1898213
Mohammadipoor A et al (2016) Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl Res 177:127–142. https://doi.org/10.1016/j.trsl.2016.06.011
Liu Z et al (2019) STC-1 ameliorates renal injury in diabetic nephropathy by inhibiting the expression of BNIP3 through the AMPK/SIRT3 pathway. Lab Invest 99:684–697. https://doi.org/10.1038/s41374-018-0176-7
Zhang Y et al (2019) Endothelial stanniocalcin 1 maintains mitochondrial bioenergetics and prevents oxidant-induced lung injury via toll-like receptor 4. Antioxid Redox Signal 30:1775–1796. https://doi.org/10.1089/ars.2018.7514
Huang L et al (2015) Stanniocalcin-1 inhibits thrombin-induced signaling and protects from bleomycin-induced lung injury. Sci Rep 5:18117. https://doi.org/10.1038/srep18117
Tang SE et al (2014) Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med 71:321–331. https://doi.org/10.1016/j.freeradbiomed.2014.03.034
Bonfante S et al (2020) Stanniocalcin-1 ameliorates cerebral ischemia by decrease oxidative stress and blood brain barrier permeability. Microvasc Res 128:103956. https://doi.org/10.1016/j.mvr.2019.103956
Bonfante S et al (2020) Stanniocalcin 1 inhibits the inflammatory response in microglia and protects against sepsis-associated encephalopathy. Neurotox Res. https://doi.org/10.1007/s12640-020-00293-y
Barialai L et al (2020) AMPK activation protects astrocytes from hypoxia–induced cell death. Int J Mol Med 45:1385–1396. https://doi.org/10.3892/ijmm.2020.4528
Waclawiczek A et al (2020) Mesenchymal niche remodeling impairs hematopoiesis via stanniocalcin 1 in acute myeloid leukemia. J Clin Invest 130:3038–3050. https://doi.org/10.1172/JCI133187
Ito Y et al (2014) Stanniocalcin-1 is induced by hypoxia inducible factor in rat alveolar epithelial cells. Biochem Biophys Res Commun 452:1091–1097. https://doi.org/10.1016/j.bbrc.2014.09.060
Yeung HY et al (2005) Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146:4951–4960. https://doi.org/10.1210/en.2005-0365
Vestergaard MB et al (2020) Higher physiological vulnerability to hypoxic exposure with advancing age in the human brain. J Cereb Blood Flow Metab 40:341–353. https://doi.org/10.1177/0271678X18818291
Gallagher CN et al (2009) The human brain utilizes lactate via the tricarboxylic acid cycle: a 13 C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132:2839–2849. https://doi.org/10.1093/brain/awp202
Bouzat P et al (2014) Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med 40:412–421. https://doi.org/10.1007/s00134-013-3203-6
Zhang K et al (2000) Stanniocalcin: a molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA 97:3637–3642. https://doi.org/10.1073/pnas.070045897
Westberg JA et al (2007) Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 38:1025–1030. https://doi.org/10.1161/01.STR.0000258113.67252.fa
Acknowledgements
This research was supported by grants from Army Medical University (Grant No. 2017XQN05), Youth Cultivation Project of PLA (Grant No. 19QNP002) and the program of Chongqing Talents.
Author information
Authors and Affiliations
Contributions
BS performed experiments, interpreted the data, and wrote the manuscript. SH, BL, GX, GE, LF, LX and WZ participated experiments. DC and JC participated in data analysis and manuscript writing. EZ and YG conceived the study design, experimental plan, and manuscript writing. All authors discussed the results and critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11064_2021_3393_MOESM2_ESM.tif
Figure S2. STC1 expression in immunohistochemcal analysis of mouse brain tissues. Supplementary material 2 (TIF 10995.7 kb)
11064_2021_3393_MOESM3_ESM.tif
Figure S3. 1 The TUNEL assay in mouse brain tissues under normoxic and 5,800-m hypobaric hypoxic conditions for 1, 3 and 7 days (n=6). Blue: DAPI; Green: GFAP; Red: TUNEL. Supplementary material 3 (TIF 9787.2 kb)
Rights and permissions
About this article
Cite this article
Sun, B., He, S., Liu, B. et al. Stanniocalcin-1 Protected Astrocytes from Hypoxic Damage Through the AMPK Pathway. Neurochem Res 46, 2948–2957 (2021). https://doi.org/10.1007/s11064-021-03393-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03393-z