Abstract
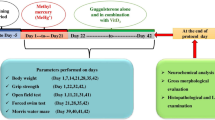
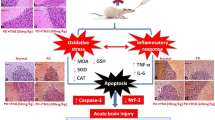
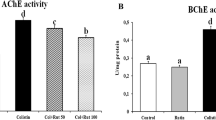
Methylmercury (MeHg) is a potent neurotoxin that causes neurotoxicity and neuronal cell death. MeHg exposure also leads to oligodendrocyte destruction, glial cell overactivation, and demyelination of motor neurons in the motor cortex and spinal cord. As a result, MeHg plays an important role in the progression of amyotrophic lateral sclerosis (ALS)-like neurocomplications. ALS is a fatal neurodegenerative disorder in which neuroinflammation is the leading cause of further CNS demyelination. Nuclear factor erythroid-2-related factor-2 (Nrf2)/Heme oxygenase-1 (HO-1) signaling pathway was thought to be a potential target for neuroprotection in ALS. Acetyl-11-keto-beta-boswellic acid (AKBA) is a multi-component pentacyclic triterpenoid mixture derived from Boswellia serrata with anti-inflammatory and antioxidant properties. The research aimed to investigate whether AKBA, as a Nrf2 / HO-1 activator, can provide protection against ALS. Thus, we explored the role of AKBA on the Nrf2/HO-1 signaling pathway in a MeHg-induced experimental ALS model. In this study, ALS was induced in Wistar rats by oral gavage of MeHg 5 mg/kg for 21 days. An open field test, force swim test, and grip strength were performed to observe experimental rats' motor coordination behaviors. In contrast, a morris water maze was performed for learning and memory. Administration of AKBA 50 mg/kg and AKBA 100 mg/kg continued from day 22 to 42. Neurochemical parameters were evaluated in the rat's brain homogenate. In the meantime, post-treatment with AKBA significantly improved behavioral, neurochemical, and gross pathological characteristics in the brain of rats by increasing the amount of Nrf2/HO-1 in brain tissue. Collectively, our findings indicated that AKBA could potentially avoid demyelination and encourage remyelination.












Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article. There are no separate or additional files.
Abbreviations
- Ach:
-
Acetylcholine
- AchE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- AKBA:
-
Acetyl-11-keto-beta-bosellic acid
- ALS:
-
Amyotrophic lateral sclerosis
- ARE:
-
Antioxidant response element
- Bcl-2:
-
B-cell lymphoma 2
- Bzip:
-
Basic leucin zipper
- Caspase-3:
-
Cysteine-aspartic proteases, cysteine aspartases or cysteine-dependent aspartate-directed proteases-3
- CAT:
-
Catalase
- FST:
-
Forced swim test
- GABA:
-
Gamma amino butyric acid
- GCL:
-
Glutamate cysteine ligase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- HD:
-
Huntington’s disease
- HO-1:
-
Hemeoxygenase 1
- IL:
-
Interleukin
- LDH:
-
Lactate dehydrogenase
- MBP:
-
Myelin basic protein
- MDA:
-
Melanodialdehyde
- MeHg:
-
Methylmercury
- MS:
-
Multiple sclerosis
- MWM:
-
Morris water maze
- NIT:
-
Nitrite
- NQO1:
-
NAD (P)H-quinone oxidoreductase 1
- Nrf2:
-
Nuclear erythroid 2 related factor
- OPC’S:
-
Oligodendrocytes
- PD:
-
Parkinson’s disease
- RNA:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SOD1:
-
Superoxide dismutase 1
- TSTQ:
-
Time spend in target quadrant
- Txnrd1:
-
Thioredoxin reductase 1
References
Hubbard JA, Szu JI, Binder DK (2018) The role of aquaporin-4 in synaptic plasticity memory and disease. Brain Res Bull 136:118–129. https://doi.org/10.1016/j.brainresbull.2017.02.011
Alam M, Yadav RK, Minj E, Tiwari A, Mehan S (2020) Exploring molecular approaches in Amyotrophic lateral sclerosis: drug targets from clinical and pre-clinical findings. Curr Mol Pharmacol. https://doi.org/10.2174/1566524020666200427214356
Gordon PH (2013) Amyotrophic Lateral Sclerosis: an update for 2013 clinical features, pathophysiology, management and therapeutic trials. Aging Dis 4:295–310
Harms MB, Baloh RH (2013) Clinical neurogenetics: amyotrophic lateral sclerosis. Neurol Clin 31:929–950. https://doi.org/10.1016/j.ncl.2013.05.003
Mhatre M, Floyd RA, Hensley K (2004) Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimer’s Dis 6:147–157. https://doi.org/10.3233/jad-2004-6206
Yadav R, Minj E, Mehan S (2021) Understanding correlation of abnormal c-JNK/p38MAPK signaling in amyotrophic lateral sclerosis: Potential drug targets and influences on neurological disorders. CNS Neurol Disord Drug Targets. https://doi.org/10.2174/1871527320666210126113848
Philips T (2013) Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 136:471–482. https://doi.org/10.1093/brain/aws339
Kang SH (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16:571–579. https://doi.org/10.1038/nn.3357
Nakamura K, Houzawa U, Uemura T (1986) Auditory brainstem responses in rats with methylmercury poisoning. Audiol Jpn 29:445–446. https://doi.org/10.1016/j.toxlet.2012.07.011
Murata K, Weihe P, Budtz-Jorgensen EJ, Grandjean PJ (2004) Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr 144:177–183. https://doi.org/10.1016/j.jpeds.2003.10.059
Dong Li Z, Proschel TC, Noble M (2007) Chemically diverse toxicants converge on Fyn and c-Cbl to disrupt precursor cell function. PLoS Biol. https://doi.org/10.1371/journal.pbio.0050035
Padhi BK, Pelletier G, Williams A, Berndt-Weis L, Yauk C, Bowers WJ, Chu I (2008) Gene expression profiling in rat cerebellum following in utero and lactational exposure to mixtures of methylmercury, polychlorinated biphenyls and organochlorine pesticides. Toxicol Lett 176:93–103. https://doi.org/10.1016/j.toxlet.2007.08.016
NRC (National Research Council) (2000) Toxicological effects of methylmercury. National Academy Press, Washington DC
Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo (2008) Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol 51:201–214. https://doi.org/10.1016/j.yrtph.2008.01.016
Farina M, Rocha JB, Aschner M (2011) Mechanisms of methylmercury induced neurotoxicity: evidence from experimental studies. Life Sci 89:555–563. https://doi.org/10.1016/j.lfs.2011.05.019
Alam MM, Minj E, Yadav RK, Mehan S (2020) Neuroprotective potential of adenyl cyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr Bioact Compd. https://doi.org/10.2174/1573407216999200723113054
Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L (2008) Neurodevelopmental toxicity of methylmercury: laboratory animal data and their contribution to human risk assessment. Regul Toxicol Pharmacol 51(2):215–229. https://doi.org/10.1016/j.yrtph.2008.03.005
Fahrion JK, Komuro Y, Li Y, Ohno N, Littner Y, Raoult E, Galas L, Vaudry D, Komuro H (2012) Rescue of neuronal migration deficits in a mouse model of fetal Minamata disease by increasing neuronal Ca2+ spike frequency. Proc Natl Acad Sci USA 109:5057–5062. https://doi.org/10.1073/pnas.1120747109
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200. https://doi.org/10.3389/fonc.2012.00200
Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1:45–49. https://doi.org/10.1016/j.redox.2012.10.001
Wang J (2018) Inflammatory cytokines and cells are potential markers for patients with cerebral apoplexy in intensive care unit. Exp Therapeutic Med 16(2):1014–1020. https://doi.org/10.3892/etm.2018.6213
Holmström KM, Baird L, Zhang Y (2013) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2:761–770. https://doi.org/10.1242/bio.20134853
Li T, Wang H, Ding Y (2014) Genetic elimination of Nrf2 aggravates secondary complications except for vasospasm after experimental subarachnoid haemorrhage in mice. Brain Res 1558:90–99. https://doi.org/10.1016/j.brainres.2014.02.036
Mitsuishi Y, Taguchi K, Kawatani Y (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22:66–79. https://doi.org/10.1016/j.ccr.2012.05.016
Lee JM, Shih AY, Murphy TH (2003) NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem 278:37948–37956. https://doi.org/10.1074/jbc.M305204200
DoNascimento JL, Oliveira KR, Crespo-Lopez ME, Macchi BM, Maues LA, Pinheiro Mda C, Silveira LC, Herculano AM (2008) Methylmercury neurotoxicity & anti-oxidant defenses. Indian J Med Res 128:373–382
Hwang GW (2012) Role of intracellular defense factors against methylmercury toxicity. Biol Pharm Bull 35:1881–1884. https://doi.org/10.1248/bpb.b212019
Feng S, Xu Z, Wang F, Yang T, Liu W, Deng Y, Xu B (2017) Sulforaphane prevents methylmercury-induced oxidative damage and excitotoxicity through activation of the Nrf2-ARE pathway. Mol Neurobiol 54:375–391. https://doi.org/10.1007/s12035-015-9643-y
Sykiotis GP, Bohmann D (2008) Keap1/Nrf2 signalling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14:76–85. https://doi.org/10.1016/j.devcel.2007.12.002
Piantadosi CA, Withers CM, Bartz RR (2011) Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286(18):16374–85. https://doi.org/10.1074/jbc.M110.207738
Cuadrado MPAIRA (2017) P 087 - NRF2 controls proteostasis through the transcriptionalregulation of autophagy. In: Proceedings of the OCC World Congress and Annual SFRR-E Conference Metabolic Stress and Redox Regulation Berlin. Free Radical Biology and Medicine 108. https://doi.org/10.1016/j.freeradbiomed.2017.04.172
Pajares M, Rojo AI, Arias E (2018) Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A (2018/06/29). Autophagy 14:1310–1322. https://doi.org/10.1080/15548627.2018.1474992
Sakata H, Niizuma K, Yoshioka H (2012) Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 32:3462–3473. https://doi.org/10.1523/JNEUROSCI.5686-11.2012
Unoki T, Akiyama M, Kumagai Y, Gonçalves FM, Farina M, Rocha JB, Aschner M (2018) Molecular pathways associated with methylmercury-induced Nrf2 modulation. Front Genet 9:373. https://doi.org/10.3389/fgene.2018.00373
Joshi G, Johnson JA (2012) The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov 7(3):218–229. https://doi.org/10.2174/157488912803252023
Sarlette A, Krampfl K, Grothe C, Neuhoff N, Dengler R, Petri S (2008) Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 67(11):1055–1062. https://doi.org/10.1097/NEN.0b013e31818b4906
Minj E, Yadav RK, Mehan S (2021) Targeting abnormal Nrf2/HO-1 signaling in amyotrophic lateral sclerosis: current Insights on drug targets and influences on neurological disorders. Curr Mol Med. https://doi.org/10.2174/1566524021666210111104920
Chien MH, Chow JM, Lee WJ (2017) Tricetin induces apoptosis of human leukemic HL-60 cells through are active oxygen species-mediated c-Jun N-Terminal kinase activation pathway. Int J Mol Sci 18(8):1667. https://doi.org/10.3390/ijms18081667
Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA (2004) Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immuno Pharmacol 4(3):429–436. https://doi.org/10.1016/j.intimp.2004.01.013
Linker RA, Lee DH, Ryan S (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of theNrf2 anti-oxidant pathway. Brain 134:678–692. https://doi.org/10.1093/brain/awq386
Van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE (2008) Severe oxidative damage in multiple sclerosis lesions coincides with enhanced anti-oxidant enzyme expression. Free Radic Biol Med 45(12):1729–1737. https://doi.org/10.1016/j.freeradbiomed.2008.09.023
Ellrichmann G, Petrasch-Parwez E, Lee DH, Reick C, Arning L, Saft C (2011) Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington’s disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0016172
Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 1147:61–69. https://doi.org/10.1196/annals.1427.036
Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278(14):12029–12038. https://doi.org/10.1074/jbc.M211558200
Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH (2005) Induction of the Nrf2-driven anti-oxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280(24):22925–36. https://doi.org/10.1074/jbc.M414635200
Cuadrado A, Rojo AI (2008) Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des 14(5):429–442. https://doi.org/10.2174/13816120878359740
Ernst E (2008) Frankincense: systematic review. BMJ. https://doi.org/10.1136/bmj.a2813
Ammon HP (2006) Boswellic acids in chronic inflammatory diseases. Planta Med 72(12):1100–1116. https://doi.org/10.1055/s-2006-947227
Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Qazi GN (2007) Boswellic acids and glucosamine show synergistic effect in preclinical anti-inflammatory study in rats. Bio Org Med Chem Lett 17(13):3706–3711. https://doi.org/10.1016/j.bmcl.2007.04.034
Doaee P, Rajaei Z, Roghani M, Alaei H, Kamalinejad M (2019) Effects of Boswellia serrataresin extract on motor dysfunction and brain oxidative stress in an experimental model of Parkinson’s disease. Avicenna J Phyto med 9(3):281–290
Wei C, Fan J, Sun X, Yao J, Guo Y, Zhou B, Shang Y (2020) Acetyl-11-keto-β-boswellic acid ameliorates cognitive deficits and reduces amyloid-β levels in APPswe/PS1dE9 mice through anti-oxidant and anti-inflammatory pathways. Free Radic Biol Med 150:96–108. https://doi.org/10.1016/j.freeradbiomed.2020.02.022
Rajabian A, Sadeghnia HR, Fanoudi S, Hosseini A (2020) Genus Boswelliaas a new candidate for neurodegenerative disorders. Iran J Basic Med Sci 23(3):277–286
Winking M, Sarikaya S, Rahmanian A, Jödicke A, Böker DK (2000) Boswellic acids inhibit glioma growth: a new treatment option? J Neurooncol 46(2):97–103. https://doi.org/10.1023/a:1006387010528
Sayed AS, Gomaa IEO, Bader M, El Sayed N (2018) Role of 3-acetyl-11-keto-beta-boswellic acid in counteracting LPS-induced neuroinflammation via modulation of miRNA-155. Mol Neuro Biol 55(7):5798–5808. https://doi.org/10.1007/s12035-017-0801-2
Girardi B, Principi M, Pricci M, Giorgio F, Annone A, Losurdo G, Ierardi E, Di Leo A, Barone M (2018) Chemoprevention of inflammation-related colorectal cancer by silymarin, acetyl-11-keto-beta-boswellic acid, curcumin and maltodextrin-enriched dietetic formulation in animal model. Carcinogenesis 39(10):1274–1282. https://doi.org/10.1093/carcin/bgy104
Kruger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Muller WE, Karas M (2008) Metabolism of boswellic acids in vitro and in vivo. Drug Metab Dispos 36(6):1135–1142. https://doi.org/10.1124/dmd.107.018424
Iram F, Khan SA, Husain A (2017) Phytochemistry and potential therapeutic actions of Boswellic acids: a mini-review. Asian Pac J Trop Biomed 7(6):513–523. https://doi.org/10.1016/j.apjtb.2017.05.001
Guedes J, Cardoso AL, Pedroso de Lima MC (2013) Involvement of microRNA in microglia-mediated immune response. Clin Dev Immunol. https://doi.org/10.1155/2013/18687
Beghelli D, Isani G, Roncada P, Andreani G, Bistoni O, Bertocchi M, Lupidi G, Alunno A (2017) Anti-oxidant and ex vivo immune system regulatory properties of Boswellia serrata extracts. Oxid Med Cell Longev 2017:7468064. https://doi.org/10.1155/2017/7468064
Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, Zhu Y, Guo C, Jia Y, Li Y, Wen A (2014) Neuroprotection by acetyl-11-keto-β-Boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep 4:7002. https://doi.org/10.1038/srep07002
Yi D, Chen M, Wang M, Li WY, Ai DW (2015) Posttreatment with 11-keto-β-boswellic acid ameliorates cerebral ischemia-reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol 52(3):1430–1439. https://doi.org/10.1007/s12035-014-8929-9
Bishnoi M, Patil CS, Shrinivas AK, Kulkarni K (2007) Co-Administration of acetyl-11-keto-β-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of cox-2 inhibitors in kainic acid induced neurotoxicity in mice. Pharmacology 79(1):34–41. https://doi.org/10.1159/000097627
Christinal J, Sumathi T (2013) Effect of Bacopa monniera extract on methylmercury-induced behavioral and histopathological changes in rats. Biol Trace Elem Res 155(1):56–64. https://doi.org/10.1007/s12011-013-9756-y
Qin Y, Li GL, Xu XH, Sun ZY, Gu JW, Gao FB (2018) Brain structure alterations and cognitive impairment following repetitive mild head impact: an in vivo MRI and behavioral study in rat. Behav Brain Res 340:41–488. https://doi.org/10.1016/j.bbr.2016.08.008
Sândor PS, Di Clemente L, Coppola G (2005) Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology 64(4):713–715. https://doi.org/10.1212/01.WNL.0000151975.03598.ED
Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J (2014) Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS ONE. https://doi.org/10.1371/journal.pone.0097423
Kumar Jha M, Ho Park D, Kook H (2016) Metabolic control of glia-mediated neuroinflammation. Curr Alzheimer Res 13(4):387–402. https://doi.org/10.2174/1567205013666151116124755
Silva-Islas CA, Chánez-Cárdenas ME, Barrera-Oviedo D, Ortiz-Plata A, Pedraza-Chaverri J, Maldonado PD (2019) Diallyl trisulfide protects rat brain tissue against the damage induced by ischemia-reperfusion through the nrf2 pathway. Antioxidants 8(9):410. https://doi.org/10.3390/antiox8090410
Li H, Lan T, Yun C, Yang K, Du Z, Luo X, Hao E, Deng J (2020) Mangiferin exerts neuroprotective activity against lead-induced toxicity and oxidative stress via Nrf2 pathway. Chin Herbal Med 12:36–46. https://doi.org/10.1016/j.chmed.2019.12.002
El-Ghaiesh SH, Bahr HI, Ibrahiem AT, Ghorab D, Alomar SY, Farag NE, Zaitone SA (2020) Metformin protects from rotenone-induced nigrostriatal neuronal death in adult mice by activating AMPK-FOXO3 signaling and mitigation of angiogenesis. Front Mol Neuro Sci 13:84. https://doi.org/10.3389/fnmol.2020.00084
Sharma N, Upadhayay S, Shandilya A, Sahu R, Singh A, Rajkhowa B, Mehan S (2021) Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomed Plus. https://doi.org/10.1016/j.phyplu.2021.100051
Rahi S, Gupta R, Sharma A, Mehan S (2021) Smo-Shh signaling activator purmorphamine ameliorates neurobehavioral, molecular, and morphological alterations in an intracerebroventricular propionic acid-induced experimental model of autism. Hum Exp Toxicol. https://doi.org/10.1177/09603271211013456
Moneim AEA (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis 30(4):935–942. https://doi.org/10.1007/s11011-015-9652-6
Mesole SB, Alfred OO, Yusuf UA, Lukubi L, Ndhlovu D (2020) Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in Wistar rats. Oxid Med Cell Longev 2:1–7. https://doi.org/10.1155/2020/8425643
Rajdev K, Siddiqui EM, Jadaun KS (2020) Mehan S (2020) Neuroprotective potential of solanesol in acombined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep 8:101–114. https://doi.org/10.1016/j.ibror.2020.03.001
Donzanti BA, Yamamoto BK (1988) An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci 43(11):913–922. https://doi.org/10.1016/0024-3205(88)90267-6
Mehan S, Rahi S, Tiwari A, Kapoor T, Rajdev K, Sharma R, Khera H, Kosey S, Kukkar U, Dudi R (2020) Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen Res 15(6):1140. https://doi.org/10.4103/1673-5374.270316
Singh S, Kumar P (2016) Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology 97(3–4):151–160. https://doi.org/10.1159/000443896
Abdel-Salam OM, Khadrawy YA, Mohammed NA (2012) Neuroprotective effect of nitric oxide donor isosorbide-dinitrate against oxidative stress induced by ethidium bromide in rat brain. EXCLI J 11:125–41
Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL (2009) Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine – a PDE1 inhibitor. Eur J Pharmacol 620(1–3):49–56. https://doi.org/10.1016/j.ejphar.2009.08.027
Kapoor T, Mehan S (2020) Neuroprotective methodologies in treatment of multiple sclerosis: current status of clinical and pre-clinical findings. Curr Drug Discov Technol. https://doi.org/10.2174/1570163817666200207100903
Cai J (2015) Progesterone alleviates acute brain injury via reducing apoptosis and oxidative stress in a rat experimental subarachnoid hemorrhage model. Neurosci Lett 600:238–243. https://doi.org/10.1016/j.neulet.2015.06.023
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Liu M, Zhang C, Liu W, Luo P, Zhang L, Wang Y, Wang Z, Fei Z (2015) A novel rat model of blast-induced traumatic brain injury simulating different damage degree: implications for morphological, neurological, and biomarker changes. Front Cell Neurosci 9:168. https://doi.org/10.3389/fncel.2015.00168
Beckmann N, Giorgetti E, Neuhaus A, Zurbruegg S, Accart N, Smith P, Perdoux J, Perrot L, Nash M, Desrayaud S, Wipfli P, Frieauff W, Shimshek DR (2018) Brain region-specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathol Commun 6(1):9. https://doi.org/10.1186/s40478-018-0510-8
Rajagopalan V, Liu Z, Allexandre D, Zhang L, Wang X-F (2013) Brain white matter shape changes in amyotrophic lateral sclerosis (ALS): a fractal dimension study. PLoS ONE. https://doi.org/10.1371/journal.pone.0073614
Tiwari A, Khera R, Rahi S, Mehan S, Makeen HA, Khormi YH, Rehman MU, Khan A (2021) Neuroprotective effect of α-mangostin in the ameliorating propionic acid-induced experimental model of autism in Wistar rats. Brain Sci 11(3):288. https://doi.org/10.3390/brainsci11030288
Kumar N, Sharma N, Khera R et al (2021) Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab Brain Dis. https://doi.org/10.1007/s11011-021-00691-x
dos Santos AA, Hort MA, Culbreth M, López-Granero C, Farina M, Rocha JBT, Aschner M (2016) Methylmercury and brain development: a review of recent literature. J Trace Elem Med Biol 38:99–107. https://doi.org/10.1016/j.jtemb.2016.03.001
Mehan S, Monga V, Rani M, Dudi R, Ghimire K (2018) Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical and cellular alterations: restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J Pharmacol 50(6):309. https://doi.org/10.4103/ijp.IJP_11_18
Matthew DR, Conrad K, Marvin E, Harvey K, Henderson D, Tawil R, Sobolewski M, Deborah A (2020) Cory-Slechta1 developmental exposure to methylmercury and resultant muscle mercury accumulation and adult motor deficits in mice. Neurotoxicology 20:30116–30119. https://doi.org/10.1016/j.neuro.2020.07.007
Cryan JF, Markou A, Lucki I (2002) Assessing anti-depressant activity in rodents: recent developments and future needs. Trends Pharma Col Sci 23:238–245. https://doi.org/10.1016/s0165-6147(02)02017-5
Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS (2007) Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neuro Sci 27:7777–7785. https://doi.org/10.1523/JNEUROSCI.0823-07.2007
Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS (2004) A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev 47(1–3):263–274. https://doi.org/10.1016/j.brainresrev.2004.05.003
Frakes AE (2014) The role of neuroinflammation in the pathogenesis of amyotrophic lateral sclerosis. Front Immunol 8:1005. https://doi.org/10.3389/fimmu.2017
Napier MD, Poole C, Satten GA, Ashley-Koch A, Marrie RA, Williamson DM (2016) Heavy metals, organic solvents, and multiple sclerosis: an exploratory look at gene-environment interactions. Arch Environ Occup Health 71(1):26–34. https://doi.org/10.1080/19338244.2014.937381
Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468:244–252. https://doi.org/10.1038/nature09614
Padhi BK, Pelletier G (2012) Perturbation of Myelin basic protein (Mbp) splice variant expression in developing rat cerebellum following perinatal exposure to methylmercury. Toxicol Lett 213:374–380. https://doi.org/10.1016/j.toxlet.2012.07.011
Kang DS, Yang YR, Lee C, Kim S, Ryu SH, Suh PG (2016) Roles of phosphoinositide-specific phospholipase Cgamma1 in brain development. Adv Biol Regul 60:167–173. https://doi.org/10.1016/j.jbior.2015.10.002
Maione AG, Brudno Y, Stojadinovic O, Park LK, Smith A, Tellechea A, Leal EC, Kearney CJ, Veves A, Tomic-Canic M (2015) Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng Part C Methods 21:499–508. https://doi.org/10.1089/ten.TEC.2014.0414
Foerster BR, Callaghan BC, Petrou M, Edden RAE, Chenevert TL, Feldman EL (2012) Decreased motor cortex γ-aminobutyric acid in amyotrophic lateral sclerosis. Neurology 78(20):1596–1600. https://doi.org/10.1212/WNL.0b013e3182563b57
Nakanishi ST, Cope TC, Rich MM, Carrasco DI, Pinter MJ (2005) Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neuro Sci 25(9):2226–2232. https://doi.org/10.1523/JNEUROSCI.5065-04.2005
Sandyk R (2006) Serotonergic mechanisms in amyotrophic lateral sclerosis. Int J Neurosci 116(7):775–826. https://doi.org/10.1080/00207450600754087
Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH (2019) Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta. https://doi.org/10.1016/j.bbagen.2019.02.001
Barber SC, Shaw PJ (2010) Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radical Biol Med 48:629–641. https://doi.org/10.1016/j.freeradbiomed.2009.11.018
Acknowledgements
The authors express their gratitude to Chairman, Mr. Parveen Garg, and Director, Dr. G.D. Gupta, ISF College of Pharmacy, Moga (Punjab), India, for their great vision and support.
Author information
Authors and Affiliations
Contributions
All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy. EM: Contribution: Clinical and pre-clinical survey. SU: Contribution: Compilation of statistical research data, revision of research manuscript. SM: Contribution: Original research hypothesis, guide, and compilation of all manuscript data. All authors approved the final version of this study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
All applicable institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minj, E., Upadhayay, S. & Mehan, S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem Res 46, 2867–2884 (2021). https://doi.org/10.1007/s11064-021-03366-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03366-2