Abstract
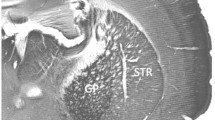
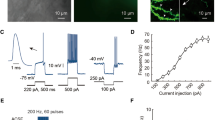
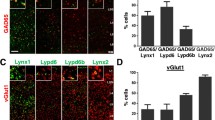
Parvalbumin-immunoreactive (Parv+) interneurons is an important component of striatal GABAergic microcircuits, which receive excitatory inputs from the cortex and thalamus, and then target striatal projection neurons. The present study aimed to examine ultrastructural synaptic connection features of Parv+ neruons with cortical and thalamic input, and striatal projection neurons by using immuno-electron microscopy (immuno-EM) and immunofluorescence techniques. Our results showed that both Parv+ somas and dendrites received numerous asymmetric synaptic inputs, and Parv+ terminals formed symmetric synapses with Parv− somas, dendrites and spine bases. Most interestingly, spine bases targeted by Parv+ terminals simultaneously received excitatory inputs at their heads. Electrical stimulation of the motor cortex (M1) induced higher proportion of striatal Parv+ neurons express c-Jun than stimulation of the parafascicular nucleus (PFN), and indicated that cortical- and thalamic-inputs differentially modulate Parv+ neurons. Consistent with that, both Parv + soma and dendrites received more VGlut1+ than VGlut2+ terminals. However, the proportion of VGlut1+ terminal targeting onto Parv+ proximal and distal dendrites was not different, but VGlut2+ terminals tended to target Parv+ somas and proximal dendrites than distal dendrites. These functional and morphological results suggested excitatory cortical and thalamic glutamatergic inputs differently modulate Parv+ interneurons, which provided inhibition inputs onto striatal projection neurons. To maintain the balance between the cortex and thalamus onto Parv+ interneurons may be an important therapeutic target for neurological disorders.






Similar content being viewed by others
References
Zhai S, Tanimura A, Graves SM et al (2018) Striatal synapses, circuits, and Parkinson’s disease. Curr Opin Neurobiol 48:9–16
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Gittis AH, Kreitzer AC (2012) Striatal microcircuitry and movement disorders. Trends Neurosci 35:557–564
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466
Gerfen CR, Keefe KA, Gauda EB (1995) D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. J Neurosci 15:8167–8176
Gerfen CR, Engber TM, Mahan LC et al (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Kawaguchi Y (1993) Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13:4908–4923
Kawaguchi Y, Wilson CJ, Augood SJ et al (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18:527–535
Tepper JM, Koos T, Wilson CJ (2004) GABAergic microcircuits in the neostriatum. Trends Neurosci 27:662–669
Straub C, Saulnier JL, Begue A et al (2016) Principles of synaptic organization of GABAergic interneurons in the striatum. Neuron 92:84–92
Silberberg G, Bolam JP (2015) Local and afferent synaptic pathways in the striatal microcircuitry. Curr Opin Neurobiol 33:182–187
Koos T, Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2:467–472
Cowan RL, Wilson CJ, Emson PC et al (1990) Parvalbumin-containing GABAergic interneurons in the rat neostriatum. J Comp Neurol 302:197–205
Marin O (2012) Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13:107–120
Mallet N, Ballion B, Le Moine C et al (2006) Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 26:3875–3884
Kitai ST, Kocsis JD, Preston RJ et al (1976) Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res 109:601–606
Dube L, Smith AD, Bolam JP (1988) Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol 267:455–471
Deschenes M, Bourassa J, Doan VD et al (1996) A single-cell study of the axonal projections arising from the posterior intralaminar thalamic nuclei in the rat. Eur J Neurosci 8:329–343
Smith AD, Bolam JP (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13:259–265
Berendse HW, Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299:187–228
Gerfen CR (1988) Synaptic organization of the striatum. J Electron Microsc Tech 10:265–281
Herkenham M, Pert CB (1981) Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature 291:415–418
Lacey CJ, Boyes J, Gerlach O et al (2005) GABA(B) receptors at glutamatergic synapses in the rat striatum. Neuroscience 136:1083–1095
Raju DV, Shah DJ, Wright TM et al (2006) Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol 499:231–243
Lei W, Jiao Y, Del MN et al (2004) Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J neurosci 24:8289–8299
Dimova R, Vuillet J, Nieoullon A et al (1993) Ultrastructural features of the choline acetyltransferase-containing neurons and relationships with nigral dopaminergic and cortical afferent pathways in the rat striatum. Neuroscience 53:1059–1071
Meredith GE, Chang HT (1994) Synaptic relationships of enkephalinergic and cholinergic neurons in the nucleus accumbens of the rat. Brain Res 667:67–76
Lapper SR, Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51:533–545
Meredith GE, Wouterlood FG (1990) Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol 296:204–221
Kachidian P, Vuillet J, Nieoullon A et al (1996) Striatal neuropeptide Y neurones are not a target for thalamic afferent fibres. Neuroreport 7:1665–1669
Vuillet J, Kerkerian L, Kachidian P et al (1989) Ultrastructural correlates of functional relationships between nigral dopaminergic or cortical afferent fibers and neuropeptide Y-containing neurons in the rat striatum. Neurosci Lett 100:99–104
Clarke DJ, Dunnett SB (1993) Synaptic relationships between cortical and dopaminergic inputs and intrinsic GABAergic systems within intrastriatal striatal grafts. J Chem Neuroanat 6:147–158
Roux L, Buzsaki G (2015) Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88:10–23
Smith Y, Raju D, Nanda B et al (2009) The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull 78:60–68
Rudkin TM, Sadikot AF (1999) Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: organization in normal striatum and effect of neonatal decortication. Neuroscience 88:1165–1175
Sidibe M, Smith Y (1999) Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience 89:1189–1208
Lapper SR, Smith Y, Sadikot AF et al (1992) Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res 580:215–224
Sciamanna G, Ponterio G, Mandolesi G et al (2015) Optogenetic stimulation reveals distinct modulatory properties of thalamostriatal vs corticostriatal glutamatergic inputs to fast-spiking interneurons. Sci Rep 5:16742
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 345:1255263
Kita H, Kosaka T, Heizmann CW (1990) Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res 536:1–15
Bennett BD, Bolam JP (1994) Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62:707–719
Schuermann M, Neuberg M, Hunter JB et al (1989) The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell 56:507–516
Herschman HR (1989) Extracellular signals, transcriptional responses and cellular specificity. Trends Biochem Sci (Amsterdam) 14:455
Herdegen T, Leah JD, Manisali A et al (1991) c-JUN-like immunoreactivity in the CNS of the adult rat: basal and transynaptically induced expression of an immediate-early gene. Neuroscience 41:643–654
Herdegen T, Kovary K, Leah J et al (1991) Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol 313:178–191
Hunt SP, Pini A, Evan G (1987) Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 328:632–634
Pearse DD, Bushell G, Leah JD (2001) Jun, Fos and Krox in the thalamus after C-fiber stimulation: coincident-input-dependent expression, expression across somatotopic boundaries, and nucleolar translocation. Neuroscience 107:143–159
Li S, Ye F, Farber JP et al (2019) Dependence of c-fos expression on amplitude of high-frequency spinal cord stimulation in a rodent model. Neuromodulation 22:172–178
Hitier M, Sato G, Zhang YF et al (2018) Effects of electrical stimulation of the rat vestibular labyrinth on c-Fos expression in the hippocampus. Neurosci Lett 677:60–64
Stiles L, Reynolds JN, Napper R et al (2018) Single neuron activity and c-Fos expression in the rat striatum following electrical stimulation of the peripheral vestibular system. Physiol Rep 6:e13791
Paxinos GWC (1986) The rat brain in stereotaxic coordinates. Elsevier Academic Press, San Diego
Kreitzer AC (2009) Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32:127–147
Mu S, OuYang L, Liu B et al (2011) Protective effect of melatonin on 3-NP induced striatal interneuron injury in rats. Neurochem Int 59:224–234
Ma Y, Zhan M, OuYang L et al (2014) The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav Brain Res 266:37–45
Mu S, Lin E, Liu B et al (2014) Melatonin reduces projection neuronal injury induced by 3-nitropropionic acid in the rat striatum. Neurodegener Dis 14:139–150
Nakano Y, Karube F, Hirai Y et al (2018) Parvalbumin-producing striatal interneurons receive excitatory inputs onto proximal dendrites from the motor thalamus in male mice. J Neurosci Res 96:1186–1207
Deng YP, Wong T, Bricker-Anthony C et al (2013) Loss of corticostriatal and thalamostriatal synaptic terminals precedes striatal projection neuron pathology in heterozygous Q140 Huntington’s disease mice. Neurobiol Dis 60:89–107
Lei W, Deng Y, Liu B et al (2013) Confocal laser scanning microscopy and ultrastructural study of VGLUT2 thalamic input to striatal projection neurons in rats. J Comp Neurol 521:1354–1377
Chen S, Yang G, Zhu Y et al (2016) A comparative study of three interneuron types in the rat spinal cord. PLoS One 11:e162969
Reiner A, Hart NM, Lei W et al (2010) Corticostriatal projection neurons—dichotomous types and dichotomous functions. Front Neuroanat 4:142
Liu B, Ouyang L, Mu S et al (2011) The morphological characteristics of corticostriatal and thalamostriatal neurons and their intrastriatal terminals in rats. Surg Radiol Anat 33:807–817
Gittis AH, Nelson AB, Thwin MT et al (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234
Gustafson N, Gireesh-Dharmaraj E, Czubayko U et al (2006) A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J Neurophysiol 95:737–752
Doig NM, Moss J, Bolam JP (2010) Cortical and thalamic innervation of direct and indirect pathway medium-sized spiny neurons in mouse striatum. J Neurosci 30:14610–14618
Goldberg JH, Tamas G, Yuste R (2003) Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J Physiol 551:49–65
Eggermann E, Jonas P (2011) How the “slow” Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci 15:20–22
Aponte Y, Bischofberger J, Jonas P (2008) Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J Physiol 586:2061–2075
Assous M, Tepper JM (2018) Excitatory extrinsic afferents to striatal interneurons and interactions with striatal microcircuitry. Eur J Neurosci 49(5):593–603
Day M, Wang Z, Ding J et al (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9:251–259
Parker PR, Lalive AL, Kreitzer AC (2016) Pathway-specific remodeling of thalamostriatal synapses in parkinsonian mice. Neuron 89:734–740
Calabresi P, Pisani A, Mercuri NB et al (1996) The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci 19:19–24
Somogyi P (1977) A specific “axo-axonal” interneuron in the visual cortex of the rat. Brain Res 136:345–350
Woo TU, Whitehead RE, Melchitzky DS et al (1998) A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA 95:5341–5346
Liu B (2011) Morphological characteristics of the striatal neural pathway by biotinylated dextran amine tracing in. Neural Regen Res 6:1931–1936
Fujiyama F, Unzai T, Nakamura K et al (2006) Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. Eur J Neurosci 24:2813–2824
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81471288), and by the National Key R&D Program of China (2017YFA0104704).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals conducted and approved by the Animal Care and Use Committee of Sun Yat-sen University (ethical permission No.: Zhongshan Medical Ethics 2014-23).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, X., Sun, L., Liu, B. et al. Morphological Study of the Cortical and Thalamic Glutamatergic Synaptic Inputs of Striatal Parvalbumin Interneurons in Rats. Neurochem Res 46, 1659–1673 (2021). https://doi.org/10.1007/s11064-021-03302-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03302-4