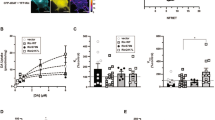
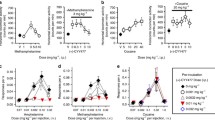
Abstract
The dopamine transporter (DAT) mediates the inactivation of released dopamine (DA) through its reuptake, and thereby plays an important homeostatic role in dopaminergic neurotransmission. Amphetamines exert their stimulant effects by targeting DAT and inducing the reverse transport of DA, leading to a dramatic increase of extracellular DA. Animal models have proven critical to investigating the molecular and cellular mechanisms underlying transporter function and its modulation by psychostimulants such as amphetamine. Here we establish a behavioral model for amphetamine action using adult Drosophila melanogaster. We use it to characterize the effects of amphetamine on sleep and sleep architecture. Our data show that amphetamine induces hyperactivity and disrupts sleep in a DA-dependent manner. Flies that do not express a functional DAT (dDAT null mutants) have been shown to be hyperactive and to exhibit significantly reduced sleep at baseline. Our data show that, in contrast to its action in control flies, amphetamine decreases the locomotor activity of dDAT null mutants and restores their sleep by modulating distinct aspects of sleep structure. To begin to explore the circuitry involved in the actions of amphetamine on sleep, we also describe the localization of dDAT throughout the fly brain, particularly in neuropils known to regulate sleep. Together, our data establish Drosophila as a robust model for studying the regulatory mechanisms that govern DAT function and psychostimulant action.






Similar content being viewed by others
References
Aggarwal S, Mortensen OV (2017) Overview of monoamine transporters. Curr Protoc Pharmacol 79:12.16.1-12.16.17. https://doi.org/10.1002/cpph.32
Hovde MJ, Larson GH, Vaughan RA, Foster JD (2019) Model systems for analysis of dopamine transporter function and regulation. Neurochem Int 123:13–21. https://doi.org/10.1016/j.neuint.2018.08.015
Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30:188–193. https://doi.org/10.1016/j.tins.2007.03.002
Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. https://doi.org/10.1016/j.neuron.2011.02.010
Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433. https://doi.org/10.1016/j.pneurobio.2005.04.003
Reith MEA, Gnegy ME (2020) Molecular mechanisms of amphetamines. Handb Exp Pharmacol 258:265–297. https://doi.org/10.1007/164_2019_251
Freyberg Z, Sonders MS, Aguilar JI et al (2016) Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun 7:10652. https://doi.org/10.1038/ncomms10652
Karam CS, Javitch JA (2018) Phosphorylation of the amino terminus of the dopamine transporter: regulatory mechanisms and implications for amphetamine action. Adv Pharmacol 82:205–234. https://doi.org/10.1016/bs.apha.2017.09.002
Asser A, Taba P (2015) Psychostimulants and movement disorders. Front Neurol 6:75. https://doi.org/10.3389/fneur.2015.00075
Cortese S (2020) Pharmacologic treatment of attention deficit-hyperactivity disorder. N Engl J Med 383:1050–1056. https://doi.org/10.1056/NEJMra1917069
Arnsten AFT (2006) Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383. https://doi.org/10.1038/sj.npp.1301164
Gainetdinov RR, Wetsel WC, Jones SR et al (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401. https://doi.org/10.1126/science.283.5400.397
Leo D, Sukhanov I, Zoratto F et al (2018) Pronounced hyperactivity, cognitive dysfunctions, and BDNF dysregulation in dopamine transporter knock-out rats. J Neurosci 38:1959–1972. https://doi.org/10.1523/JNEUROSCI.1931-17.2018
Efimova EV, Gainetdinov RR, Budygin EA, Sotnikova TD (2016) Dopamine transporter mutant animals: a translational perspective. J Neurogenet 30:5–15. https://doi.org/10.3109/01677063.2016.1144751
Hall FS, Sora I, Hen R, Uhl GR (2014) Serotonin/dopamine interactions in a hyperactive mouse: reduced serotonin receptor 1B activity reverses effects of dopamine transporter knockout. PLoS ONE 9:e115009. https://doi.org/10.1371/journal.pone.0115009
Pörzgen P, Park SK, Hirsh J et al (2001) The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol Pharmacol 59:83–95. https://doi.org/10.1124/mol.59.1.83
Neckameyer WS, White K (1993) Drosophila tyrosine hydroxylase is encoded by the pale locus. J Neurogenet 8:189–199. https://doi.org/10.3109/01677069309083448
Livingstone MS, Tempel BL (1983) Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature 303:67–70. https://doi.org/10.1038/303067a0
Greer CL, Grygoruk A, Patton DE et al (2005) A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol 64:239–258. https://doi.org/10.1002/neu.20146
Karam CS, Jones SK, Javitch JA (2020) Come Fly with Me: an overview of dopamine receptors in Drosophila melanogaster. Basic Clin Pharmacol Toxicol 126(Suppl 6):56–65. https://doi.org/10.1111/bcpt.13277
Yamamoto S, Seto ES (2014) Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim 63:107–119
Nall A, Sehgal A (2014) Monoamines and sleep in Drosophila. Behav Neurosci 128:264–272. https://doi.org/10.1037/a0036209
Alekseyenko OV, Chan Y-B, Li R, Kravitz EA (2013) Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA 110:6151–6156. https://doi.org/10.1073/pnas.1303446110
van Swinderen B (2011) Attention in Drosophila. Int Rev Neurobiol 99:51–85. https://doi.org/10.1016/B978-0-12-387003-2.00003-3
Waddell S (2013) Reinforcement signalling in Drosophila; dopamine does it all after all. Curr Opin Neurobiol 23:324–329. https://doi.org/10.1016/j.conb.2013.01.005
Kaun KR, Azanchi R, Maung Z et al (2011) A Drosophila model for alcohol reward. Nat Neurosci 14:612–619. https://doi.org/10.1038/nn.2805
Waddell S (2010) Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci 33:457–464. https://doi.org/10.1016/j.tins.2010.07.001
Kaun KR, Rothenfluh A (2017) Dopaminergic rules of engagement for memory in Drosophila. Curr Opin Neurobiol 43:56–62. https://doi.org/10.1016/j.conb.2016.12.011
Beckwith EJ, French AS (2019) Sleep in drosophila and its context. Front Physiol 10:1167. https://doi.org/10.3389/fphys.2019.01167
Ly S, Pack AI, Naidoo N (2018) The neurobiological basis of sleep: insights from Drosophila. Neurosci Biobehav Rev 87:67–86. https://doi.org/10.1016/j.neubiorev.2018.01.015
Shaw PJ, Cirelli C, Greenspan RJ, Tononi G (2000) Correlates of sleep and waking in Drosophila melanogaster. Science 287:1834–1837. https://doi.org/10.1126/science.287.5459.1834
Hendricks JC, Finn SM, Panckeri KA et al (2000) Rest in Drosophila is a sleep-like state. Neuron 25:129–138. https://doi.org/10.1016/s0896-6273(00)80877-6
Nitz DA, van Swinderen B, Tononi G, Greenspan RJ (2002) Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol 12:1934–1940. https://doi.org/10.1016/s0960-9822(02)01300-3
Bushey D, Tononi G, Cirelli C (2015) Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc Natl Acad Sci USA 112:4785–4790. https://doi.org/10.1073/pnas.1419603112
Kume K, Kume S, Park SK et al (2005) Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25:7377–7384. https://doi.org/10.1523/JNEUROSCI.2048-05.2005
Ueno T, Kume K (2014) Functional characterization of dopamine transporter in vivo using Drosophila melanogaster behavioral assays. Front Behav Neurosci 8:303. https://doi.org/10.3389/fnbeh.2014.00303
van der Voet M, Harich B, Franke B, Schenck A (2016) ADHD-associated dopamine transporter, latrophilin and neurofibromin share a dopamine-related locomotor signature in Drosophila. Mol Psychiatry 21:565–573. https://doi.org/10.1038/mp.2015.55
Pizzo AB, Karam CS, Zhang Y et al (2014) Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry 19:279–281. https://doi.org/10.1038/mp.2013.29
Pizzo AB, Karam CS, Zhang Y et al (2013) The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry 18:824–833. https://doi.org/10.1038/mp.2012.82
Andretic R, van Swinderen B, Greenspan RJ (2005) Dopaminergic modulation of arousal in Drosophila. Curr Biol 15:1165–1175. https://doi.org/10.1016/j.cub.2005.05.025
van Swinderen B, Brembs B (2010) Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci 30:1003–1014. https://doi.org/10.1523/JNEUROSCI.4516-09.2010
Bainton RJ, Tsai LT, Singh CM et al (2000) Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10:187–194. https://doi.org/10.1016/s0960-9822(00)00336-5
McClung C, Hirsh J (1998) Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol 8:109–112. https://doi.org/10.1016/s0960-9822(98)70041-7
Hamilton PJ, Belovich AN, Khelashvili G et al (2014) PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol 10:582–589. https://doi.org/10.1038/nchembio.1545
Belovich AN, Aguilar JI, Mabry SJ et al (2019) A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol Psychiatry. https://doi.org/10.1038/s41380-019-0620-0
Pfeiffenberger C, Lear BC, Keegan KP, Allada R (2010) Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) system. Cold Spring Harb Protoc 2010:pdb.prot5518. https://doi.org/10.1101/pdb.prot5518
Cichewicz K, Garren EJ, Adiele C et al (2017) A new brain dopamine-deficient Drosophila and its pharmacological and genetic rescue. Genes Brain Behav 16:394–403. https://doi.org/10.1111/gbb.12353
Riemensperger T, Isabel G, Coulom H et al (2011) Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci USA 108:834–839. https://doi.org/10.1073/pnas.1010930108
Shang Y, Haynes P, Pírez N et al (2011) Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 14:889–895. https://doi.org/10.1038/nn.2860
Andretic R, Shaw PJ (2005) Essentials of sleep recordings in Drosophila: moving beyond sleep time. Meth Enzymol 393:759–772. https://doi.org/10.1016/S0076-6879(05)93040-1
Mao Z, Davis RL (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3:5. https://doi.org/10.3389/neuro.04.005.2009
Xie T, Ho MCW, Liu Q et al (2018) A genetic toolkit for dissecting dopamine circuit function in drosophila. Cell Rep 23:652–665. https://doi.org/10.1016/j.celrep.2018.03.068
Scheffer LK, Xu CS, Januszewski M et al (2020) A connectome and analysis of the adult Drosophila central brain. Elife. https://doi.org/10.7554/eLife.57443
Hartenstein V, Cruz L, Lovick JK, Guo M (2017) Developmental analysis of the dopamine-containing neurons of the Drosophila brain. J Comp Neurol 525:363–379. https://doi.org/10.1002/cne.24069
Artiushin G, Sehgal A (2017) The Drosophila circuitry of sleep-wake regulation. Curr Opin Neurobiol 44:243–250. https://doi.org/10.1016/j.conb.2017.03.004
Meissner GW, Nern A, Singer RH et al (2019) Mapping neurotransmitter identity in the whole-mount drosophila brain using multiplex high-throughput fluorescence in situ hybridization. Genetics 211:473–482. https://doi.org/10.1534/genetics.118.301749
Thimgan MS, Berg JS, Stuart AE (2006) Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol 209:3383–3404. https://doi.org/10.1242/jeb.02328
Croset V, Treiber CD, Waddell S (2018) Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. Elife. https://doi.org/10.7554/eLife.34550
Penmatsa A, Wang KH, Gouaux E (2013) X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503:85–90. https://doi.org/10.1038/nature12533
Joiner WJ, Crocker A, White BH, Sehgal A (2006) Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441:757–760. https://doi.org/10.1038/nature04811
Pitman JL, McGill JJ, Keegan KP, Allada R (2006) A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441:753–756. https://doi.org/10.1038/nature04739
Aso Y, Hattori D, Yu Y et al (2014) The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3:e04577. https://doi.org/10.7554/eLife.04577
Aso Y, Siwanowicz I, Bräcker L et al (2010) Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol 20:1445–1451. https://doi.org/10.1016/j.cub.2010.06.048
Cervantes-Sandoval I, Phan A, Chakraborty M, Davis RL (2017) Reciprocal synapses between mushroom body and dopamine neurons form a positive feedback loop required for learning. Elife. https://doi.org/10.7554/eLife.23789
Takemura S-Y, Aso Y, Hige T et al (2017) A connectome of a learning and memory center in the adult Drosophila brain. Elife. https://doi.org/10.7554/eLife.26975
Deng B, Li Q, Liu X et al (2019) Chemoconnectomics: mapping chemical transmission in drosophila. Neuron 101:876-893.e4. https://doi.org/10.1016/j.neuron.2019.01.045
Kahsai L, Carlsson MA, Winther AME, Nässel DR (2012) Distribution of metabotropic receptors of serotonin, dopamine, GABA, glutamate, and short neuropeptide F in the central complex of Drosophila. Neuroscience 208:11–26. https://doi.org/10.1016/j.neuroscience.2012.02.007
Boto T, Stahl A, Zhang X et al (2019) Independent contributions of discrete dopaminergic circuits to cellular plasticity, memory strength, and valence in drosophila. Cell Rep 27:2014-2021.e2. https://doi.org/10.1016/j.celrep.2019.04.069
Strausfeld NJ, Hirth F (2013) Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340:157–161. https://doi.org/10.1126/science.1231828
Wolff T, Rubin GM (2018) Neuroarchitecture of the Drosophila central complex: a catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J Comp Neurol 526:2585–2611. https://doi.org/10.1002/cne.24512
Liu S, Liu Q, Tabuchi M, Wu MN (2016) Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell 165:1347–1360. https://doi.org/10.1016/j.cell.2016.04.013
Donlea JM, Pimentel D, Miesenböck G (2014) Neuronal machinery of sleep homeostasis in drosophila. Neuron 81:1442. https://doi.org/10.1016/j.neuron.2014.03.008
Liu Q, Liu S, Kodama L et al (2012) Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol 22:2114–2123. https://doi.org/10.1016/j.cub.2012.09.008
Ueno T, Tomita J, Tanimoto H et al (2012) Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci 15:1516–1523. https://doi.org/10.1038/nn.3238
Kong EC, Woo K, Li H et al (2010) A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE 5:e9954. https://doi.org/10.1371/journal.pone.0009954
Omoto JJ, Nguyen B-CM, Kandimalla P et al (2018) Neuronal constituents and putative interactions within the drosophila ellipsoid body neuropil. Front Neural Circuits 12:103. https://doi.org/10.3389/fncir.2018.00103
Suver MP, Matheson AMM, Sarkar S et al (2019) Encoding of wind direction by central neurons in drosophila. Neuron 102:828-842.e7. https://doi.org/10.1016/j.neuron.2019.03.012
Coates KE, Calle-Schuler SA, Helmick LM et al (2020) The wiring logic of an identified serotonergic neuron that spans sensory networks. J Neurosci 40:6309–6327. https://doi.org/10.1523/JNEUROSCI.0552-20.2020
Barr AM, Lehmann-Masten V, Paulus M et al (2004) The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology 29:221–228. https://doi.org/10.1038/sj.npp.1300343
Xu T-X, Ma Q, Spealman RD, Yao W-D (2010) Amphetamine modulation of long-term potentiation in the prefrontal cortex: dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant. J Neurochem 115:1643–1654. https://doi.org/10.1111/j.1471-4159.2010.07073.x
Del’Guidice T, Lemasson M, Etiévant A et al (2014) Dissociations between cognitive and motor effects of psychostimulants and atomoxetine in hyperactive DAT-KO mice. Psychopharmacology 231:109–122. https://doi.org/10.1007/s00213-013-3212-8
Demchyshyn LL, Pristupa ZB, Sugamori KS et al (1994) Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci USA 91:5158–5162. https://doi.org/10.1073/pnas.91.11.5158
Giang T, Ritze Y, Rauchfuss S et al (2011) The serotonin transporter expression in Drosophila melanogaster. J Neurogenet 25:17–26. https://doi.org/10.3109/01677063.2011.553002
Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50:447–477. https://doi.org/10.1146/annurev.ento.50.071803.130404
Niens J, Reh F, Çoban B et al (2017) Dopamine modulates serotonin innervation in the drosophila brain. Front Syst Neurosci 11:76. https://doi.org/10.3389/fnsys.2017.00076
Agosto J, Choi JC, Parisky KM et al (2008) Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci 11:354–359. https://doi.org/10.1038/nn2046
Geissmann Q, Garcia Rodriguez L, Beckwith EJ, Gilestro GF (2019) Rethomics: an R framework to analyse high-throughput behavioural data. PLoS ONE 14:e0209331. https://doi.org/10.1371/journal.pone.0209331
Wickham H (2011) ggplot2. WIREs Comp Stat 3:180–185. https://doi.org/10.1002/wics.147
Kassambara A (2020) ggpubr: “ggplot2” based publication ready plots. CRAN
Reymann J (2018) LIGHTNING: image information extraction by adaptive deconvolution [White paper]. Leica Microsystems
Acknowledgements
This work was supported by R01 DA041510 (JAJ and CSK), U01 DA042233 (JAJ, CSK and BLW), and T32 MH018870 (SKJ). We thank Dr. Eric Gouaux for providing the anti-dDAT antibody, Dr. Stephen Thornquist for advice on using the antibody for fluorescence imaging, Dr. Claudia Ma for her help with the initial experimental setup, and Dr. Mimi Shirazu-Hiza for sharing her Trikinetic monitors during the initial setup of the assay.
Author information
Authors and Affiliations
Contributions
CSK and JAJ designed the experiments. CSK, BLW, SKJ, and JAJ analyzed the data, discussed the results, and wrote the manuscript. CSK developed the behavioral assay and conducted the behavioral experiments. BLW performed the analysis of activity, sleep, and sleep structure data, in addition to all statistical analyses. BLW and SKJ performed the immunostaining and imaging analysis.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Prof. Baruch Kanner.
Supplementary Information
Below is the link to the electronic supplementary material.
11064_2021_3275_MOESM1_ESM.jpg
Supplementary file1 (JPG 1046 KB)
Supplementary Image 1. Maximum intensity projection of neuronal cell bodies, as well as parts of the PCB and IB, in whole adult fly brains stained with anti-dDAT antibody (green), imaged under 63 × with a 3.5 × optical zoom. Scale bar indicates 10 µm. LIGHTNING adaptive deconvolution was used to obtain maximum resolution [90].
Supplementary file2 (MP4 67941 KB)
Supplementary Video 1. 3D reconstruction of an anterior segment consisting of 122 sections of 0.3-µm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63 ×.
Supplementary file3 (MP4 105947 KB)
Supplementary Video 2. 3D reconstruction of a posterior segment consisting of 175 sections of 0.3-µm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63 ×.
Supplementary file4 (MP4 20588 KB)
Supplementary Video 3. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) in Drosophila adult brain captured at 40 ×.
Supplementary file5 (MP4 112137 KB)
Supplementary Video 4. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) captured at 63 ×.
Rights and permissions
About this article
Cite this article
Karam, C.S., Williams, B.L., Jones, S.K. et al. The Role of the Dopamine Transporter in the Effects of Amphetamine on Sleep and Sleep Architecture in Drosophila. Neurochem Res 47, 177–189 (2022). https://doi.org/10.1007/s11064-021-03275-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03275-4