Abstract
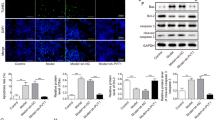
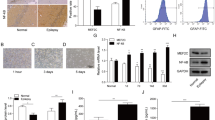
This study aimed to explore the effects and function of microRNA-101a-3p (miR-101a-3p) in epilepsy. Rat model of pilocarpine-induced epilepsy was established and the seizure frequency was recorded. Expression of miR-101a-3p and c-Fos in hippocampus tissues of Rat models were detected by qRT-PCR and western blot. Besides, we established a hippocampal neuronal culture model of acquired epilepsy using Mg2+ free medium to evaluate the effects of miR-101a-3p and c-Fos in vitro. Cells were transfected with miR-101a-3p mimic, si-c-FOS, miR-101a-3p + c-FOS and its corresponding controls. MTT assay was used to detect cell viability upon transfection. Flow cytometry was performed to determine the apoptosis rate. Western blot was performed to measure the protein expression of apoptosis-related proteins (Bcl-2, Bax, and cleaved caspase 3), autophagy-related proteins (LC3 and Beclin1) and c-FOS. The targeting relationship between miR-101a-3p and c-FOS was predicted and verified by TargetScan software and dual-luciferase reporter assay. The role of miR-101a-3p was validated using epilepsy rat models in vivo. Another Rat models of pilocarpine-induced epilepsy with miR-NC or miR-101a-3p injection were established to evaluate the effect of miR-101a-3p overexpression on epilepsy in vivo. MiR-101a-3p was downregulated while c-FOS was increased in hippocampus tissues of Rat model of pilocarpine-induced epilepsy. Overexpression of miR-101a-3p or c-FOS depletion promoted cell viability, inhibited cell apoptosis and autophagy. C-FOS was a target of miR-101a-3p and miR-101a-3p negatively regulated c-FOS expression to function in epilepsy. Overexpression of miR-101a-3p attenuated pilocarpine-induced epilepsy in Rats in vivo. This study indicated that miR-101a-3p could attenuate pilocarpine-induced epilepsy by repressing c-Fos expression.






Similar content being viewed by others
References
Singh A, Trevick S (2016) The epidemiology of global epilepsy. Neurol Clin 34:837–847
Trinka E, Kwan P, Lee B, Dash A (2018) Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia 60:7
Fauser S, Tumani H (2017) Epilepsy. Handb Clin Neurol 146:259–266
Cendes F, Sakamoto AC, Spreafico R, Bingaman W, Becker AJ (2014) Epilepsies associated with hippocampal sclerosis. Acta Neuropathol 128:21–37
Karnati HK, Panigrahi MK, Gutti RK, Greig NH, Tamargo IA (2015) miRNAs: key players in neurodegenerative disorders and epilepsy. J Alzheimer’s Dis 48:563–580
Kortvelyessy P, Huchtemann T, Heinze HJ, Bittner DM (2017) Progranulin and its related micrornas after status epilepticus: possible mechanisms of neuroprotection. Int J Mol Sci 18:490
Avansini SH, de Sousa Lima BP, Secolin R, Santos ML, Coan AC, Vieira AS, Torres FR, Carvalho BS, Alvim MK, Morita ME, Yasuda CL, Pimentel-Silva LR, Dogini DB, Rogerio F, Cendes F, Lopes-Cendes I (2017) MicroRNA hsa-miR-134 is a circulating biomarker for mesial temporal lobe epilepsy. PLoS ONE 12:e0173060
Zheng H, Tang R, Yao Y, Ji Z, Cao Y, Liu Z, Peng F, Wang W, Can D, Xing H, Bu G, Xu H, Zhang YW, Zheng W (2016) MiR-219 protects against seizure in the kainic acid model of epilepsy. Mol Neurobiol 53:1–7
Vilardo E, Barbato C, Ciotti M, Cogoni C, Ruberti F (2010) MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J Biol Chem 285:18344–18351
Wu Q, Yi X (2018) Down-regulation of long noncoding RNA MALAT1 protects hippocampal neurons against excessive autophagy and apoptosis via the PI3K/Akt signaling pathway in rats with epilepsy. J Mol Neurosci 65:234–245
Cohen JL, Ata AE, Jackson NL, Rahn EJ, Ramaker RC, Cooper S, Kerman IA, Clinton SM (2017) Differential stress induced c-Fos expression and identification of region-specific miRNA-mRNA networks in the dorsal raphe and amygdala of high-responder/low-responder rats. Behav Brain Res 319:110–123
Herrera DG, Robertson HA (1996) Activation of c-fos in the brain. Prog Neurobiol 50:83–107
Watanabe Y, Johnson RS, Butler LS, Binder DK, Spiegelman BM, Papaioannou VE, McNamara JO (1996) Null mutation of c-fos impairs structural and functional plasticities in the kindling model of epilepsy. J Neurosci 16:3827–3836
Hoffman GE, Smith MS, Verbalis JG (1993) c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14:173–213
Dragunow M, Faull R (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29:261–265
André V, Pineau N, Motte JE, Marescaux C, Nehlig A (1998) Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur J Neurosci 10:2094–2106
Malhi SM, Jawed H, Hanif F, Ashraf N, Zubair F, Siddiqui BS, Begum S, Kabir N, Simjee SU (2014) Modulation of c-Fos and BDNF protein expression in pentylenetetrazole-kindled mice following the treatment with novel antiepileptic compound HHL-6. Biomed Res Int 2014:876712
Wu DM, Zhang YT, Lu J, Zheng YL (2018) Effects of microRNA-129 and its target gene c-Fos on proliferation and apoptosis of hippocampal neurons in rats with epilepsy via the MAPK signaling pathway. J Cell Physiol 233:6632–6643
Lin B, Xu J, Wang F, Wang J, Zhao H, Feng D (2020) LncRNA XIST promotes myocardial infarction by regulating FOS through targeting miR-101a-3p. Aging 12:7232–7247
Geng JF, Liu X, Zhao HB, Fan WF, Geng JJ, Liu XZ (2018) LncRNA UCA1 inhibits epilepsy and seizure-induced brain injury by regulating miR-495/Nrf2-ARE signal pathway. Int J Biochem Cell Biol 99:133–139
Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Loscher W (2001) Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res 46:111–119
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Wang W, Wang X, Chen L, Zhang Y, Xu Z, Liu J, Jiang G, Li J, Zhang X, Wang K, Wang J, Chen G, Luo J (2016) The microRNA miR-124 suppresses seizure activity and regulates CREB1 activity. Expert Rev Mol Med 18:e4
Xiao Z, Peng J, Wu L, Arafat A, Yin F (2017) The effect of IL-1beta on synaptophysin expression and electrophysiology of hippocampal neurons through the PI3K/Akt/mTOR signaling pathway in a rat model of mesial temporal lobe epilepsy. Neurol Res 39:640–648
Xu Z, Zhang J, Lei X, Xu Z, Peng Y, Yao B, Xu P (2013) Effects of valproate sodium on extracellular signal-regulated kinase 1/2 phosphorylation following hippocampal neuronal epileptiform discharge in rats. Exp Ther Med 6:1397–1401
Nowak JS, Michlewski G (2013) miRNAs in development and pathogenesis of the nervous system. Biochem Soc Trans 41:815–820
Li MM, Li XM, Zheng XP, Yu JT, Tan L (2014) MicroRNAs dysregulation in epilepsy. Brain Res 1584:94–104
Schreiber SS, Tocco G, Najm I, Finch CE, Johnson SA, Baudry M (1992) Absence of c-fos induction in neonatal rat brain after seizures. Neurosci Lett 136:31–35
D’Intino G, Vaccari F, Sivilia S, Scagliarini A, Gandini G, Giardino L, Calzà L (2006) A molecular study of hippocampus in dogs with convulsion during canine distemper virus encephalitis. Brain Res 1098:186–195
Sinel'nikova VV, Shubina LV, Gol'tiaev MV, Loseva EV, Kichigina VF (2012) [Detection of c-fos expression in animal brain in a pilocarpine model of temporal lobe epilepsy]. Zhurnal vysshei nervnoi deiatelnosti imeni I P Pavlova 62:497–505
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geng, J., Zhao, H., Liu, X. et al. MiR-101a-3p Attenuated Pilocarpine-Induced Epilepsy by Downregulating c-FOS. Neurochem Res 46, 1119–1128 (2021). https://doi.org/10.1007/s11064-021-03245-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03245-w