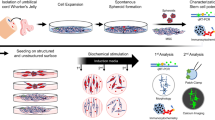
Abstract
Neurodegenerative disorders present a broad group of neurological diseases and remain one of the greatest challenges and burdens to mankind. Maladies like amyotrophic lateral sclerosis, Alzheimer’s disease, stroke or spinal cord injury commonly features astroglia involvement (astrogliosis) with signs of inflammation. Regenerative, paracrine and immunomodulatory properties of human mesenchymal stromal cells (hMSCs) could target the above components, thus opening new therapeutic possibilities for regenerative medicine. A special interest should be given to hMSCs derived from the umbilical cord (UC) tissue, due to their origin, properties and lack of ethical paradigms. The aim of this study was to establish standard operating and scale-up good manufacturing practice (GMP) protocols of UC-hMSCs isolation, characterization, expansion and comparison of cells’ properties when harvested on T-flasks versus using a large-scale bioreactor system. Human UC-hMSCs, isolated by tissue explant culture technique from Wharton’s jelly, were harvested after reaching 75% confluence and cultured using tissue culture flasks. Obtained UC-hMSCs prior/after the cryopreservation and after harvesting in a bioreactor, were fully characterized for “mesenchymness” immunomodulatory, tumorigenicity and genetic stability, senescence and cell-doubling properties, as well as gene expression features. Our study demonstrates an efficient and simple technique for large scale UC-hMSCs expansion. Harvesting of UC-hMSCs’ using classic and large scale methods did not alter UC-hMSCs’ senescence, genetic stability or in vitro tumorigenicity features. We observed comparable growth and immunomodulatory capacities of fresh, frozen and expanded UC-hMSCs. We found no difference in the ability to differentiate toward adipogenic, osteogenic and chondrogenic lineages between classic and large scale UC-hMSCs expansion methods. Both, methods enabled derivation of genetically stabile cells with typical mesenchymal features. Interestingly, we found significantly increased mRNA expression levels of neural growth factor (NGF) and downregulated insulin growth factor (IGF) in UC-hMSCs cultured in bioreactor, while IL4, IL6, IL8, TGFb and VEGF expression levels remained at the similar levels. A culturing of UC-hMSCs using a large-scale automated closed bioreactor expansion system under the GMP conditions does not alter basic “mesenchymal” features and quality of the cells. Our study has been designed to pave a road toward translation of basic research data known about human UC-MSCs for the future clinical testing in patients with neurological and immunocompromised disorders. An industrial manufacturing of UC-hMSCs next will undergo regulatory approval following advanced therapy medicinal products (ATMP) criteria prior to clinical application and approval to be used in patients.






Similar content being viewed by others
References
Heneka MT, Carson MJ, El Khoury J et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405
Rehorova M, Vargova I, Forostyak S et al (2019) A combination of intrathecal and intramuscular application of human mesenchymal stem cells partly reduces the activation of necroptosis in the spinal cord of SOD1(G93A) rats. Stem Cells Transl Med 8:535–547
Forostyak S, Homola A, Turnovcova K et al (2014) Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells 32:3163–3172
Cui Y, Ma S, Zhang C et al (2017) Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res 320:291–301
Can A, Celikkan FT, Cinar O (2017) Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy 19:1351–1382
Kalaszczynska I, Ferdyn K (2015) Wharton’s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int 2015:430847
Forostyak S, Jendelova P, Sykova E (2013) The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 95:2257–2270
Troyer DL, Weiss ML (2008) Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 26:591–599
Yoon JH, Roh EY, Shin S et al (2013) Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int 2013:428726
Tong CK, Vellasamy S, Tan BC et al (2011) Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int 35:221–226
Li J, Xu SQ, Zhao YM et al (2018) Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep 18:4969–4977
Donders R, Bogie JFJ, Ravanidis S et al (2018) Human Wharton’s jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared to bone marrow-derived stem cells. Stem Cells Dev 27:65–84
Wang Q, Yang Q, Wang Z et al (2016) Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum Vacc Immunother 12:85–96
Kim DW, Staples M, Shinozuka K et al (2013) Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 14:11692–11712
Capelli C, Gotti E, Morigi M et al (2011) Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy 13:786–801
Gauthaman K, Fong CY, Suganya CA et al (2012) Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod Biomed Online 24:235–246
Rachakatla RS, Marini F, Weiss ML et al (2007) Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther 14:828–835
Bunpetch V, Wu H, Zhang S et al (2017) From “bench to bedside”: current advancement on large-scale production of mesenchymal stem cells. Stem Cells Dev 26:1662–1673
Russell AL, Lefavor RC, Zubair AC (2018) Characterization and cost-benefit analysis of automated bioreactor-expanded mesenchymal stem cells for clinical applications. Transfusion 58:2374–2382
Savelli S, Trombi L, D’Alessandro D et al (2018) Pooled human serum: a new culture supplement for bioreactor-based cell therapies. Prelim Results Cytother 20:556–563
Barckhausen C, Rice B, Baila S et al (2016) GMP-compliant expansion of clinical-grade human mesenchymal stromal/stem cells using a closed hollow fiber bioreactor. Methods Mol Biol 1416:389–412
Fekete N, Rojewski MT, Furst D et al (2012) GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS ONE 7:e43255
Nehybova T, Smarda J, Daniel L et al (2015) Wedelolactone induces growth of breast cancer cells by stimulation of estrogen receptor signalling. J Steroid Biochem Mol Biol 152:76–83
Tichy M, Knopfova L, Jarkovsky J et al (2016) Overexpression of c-Myb is associated with suppression of distant metastases in colorectal carcinoma. Tumour Biol 37:10723–10729
Uccelli A, Laroni A, Brundin L et al (2019) MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): a randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 20:263
Sykova E, Rychmach P, Drahoradova I et al (2017) Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: results of phase I/IIa clinical trial. Cell Transpl 26:647–658
Vaquero J, Zurita M, Rico MA et al (2018) Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy 20:806–819
Lindvall O, Kokaia Z (2009) Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci 30:260–267
El Omar R, Beroud J, Stoltz JF et al (2014) Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng B 20:523–544
Lambrechts T, Sonnaert M, Schrooten J et al (2016) Large-scale mesenchymal stem/stromal cell expansion: a visualization tool for bioprocess comparison. Tissue Eng B 22:485–498
Bunpetch V, Zhang ZY, Zhang X et al (2019) Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials 196:67–79
Hupfeld J, Gorr IH, Schwald C et al (2014) Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng 111:2290–2302
Mizukami A, Fernandes-Platzgummer A, Carmelo JG et al (2016) Stirred tank bioreactor culture combined with serum-/xenogeneic-free culture medium enables an efficient expansion of umbilical cord-derived mesenchymal stem/stromal cells. Biotechnol J 11:1048–1059
Tozetti PA, Caruso SR, Mizukami A et al (2017) Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions. Biotechnol Prog 33:1358–1367
Rojewski MT, Fekete N, Baila S et al (2013) GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transpl 22:1981–2000
Carmelo JG, Fernandes-Platzgummer A, Diogo MM et al (2015) A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol J 10:1235–1247
Li R, Wu Y, Zou S et al (2017) NGF attenuates high glucose-induced er stress, preventing schwann cell apoptosis by activating the PI3K/Akt/GSK3beta and ERK1/2 pathways. Neurochem Res 42:3005–3018
Eyjolfsdottir H, Eriksdotter M, Linderoth B et al (2016) Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alzheimers Res Ther 8:30
Wagner W, Ho AD, Zenke M (2010) Different facets of aging in human mesenchymal stem cells. Tissue Eng B 16:445–453
Li Y, Wu Q, Wang Y et al (2017) Senescence of mesenchymal stem cells (review). Int J Mol Med 39:775–782
Naji A, Eitoku M, Favier B et al (2019) Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 76:3323–3348
Wang LT, Ting CH, Yen ML et al (2016) Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci 23:76
Acknowledgements
Supported by Technology Agency, Czech Republic (TACR) Program DELTA, project TF03000037 and The Ministry of Industry and Trade of the CR (MPO), Aplikace (III. výzva) project CZ.01.1.02/0.0/0.0/16_084/0010317.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
All umbilical cord samples were collected at the Department of Obstetrics and Gynecology, University Hospital Brno with informed consent from mothers and approval from Ethics Committee of University Hospital Brno.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honour of Professor Eva Sykova.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vymetalova, L., Kucirkova, T., Knopfova, L. et al. Large-Scale Automated Hollow-Fiber Bioreactor Expansion of Umbilical Cord-Derived Human Mesenchymal Stromal Cells for Neurological Disorders. Neurochem Res 45, 204–214 (2020). https://doi.org/10.1007/s11064-019-02925-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02925-y