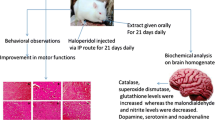
Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder associated with oxidative stress. Therefore, finding new antioxidant sources might be beneficial for its treatment. Avocado Persea americana is a fruit widely cultivated in tropical and subtropical climates worldwide. Although avocado by-products in the form of peel, seed coat and seeds are currently of no commercial use, they constitute a natural source of bioactive compounds. Methanolic (80%) extract obtained from lyophilized ground peels, seed coats, and seeds of the avocado Hass, Fuerte, Reed and Colinred varieties were analyzed for their total phenolic content (TPC) and their correlations with antioxidant capacity (AC) were assessed by ABTS, FRAP, and ORAC assays. For all varieties, the var. Colinred peel shows the highest TPC and AC. Further analysis showed that the var. Colinred peel presented major phenolic compounds B-type procyanidins and epicatechin according to HPLC–MS. The antioxidant effect of peel extract was evaluated upon in vivo oxidative stress (OS) model. We show for the first time that the peel extract can protect and/or prevent transgenic parkinDrosophila melanogaster fly against paraquat-induced OS, movement impairment and lipid peroxidation, as model of PD. Our findings offer an exceptional opportunity to test natural disease-modifying substances from avocado’s by-products.






Similar content being viewed by others
References
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson's disease. J Neurochem 139(Suppl 1):318–324. https://doi.org/10.1111/jnc.13691
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson's disease. Front Neuroanat 9:91. https://doi.org/10.3389/fnana.2015.00091
Coles LD, Tuite PJ, Öz G, Mishra UR, Kartha RV, Sullivan KM, Cloyd JC, Terpstra M (2018) Repeated-dose oral N-Acetylcysteine in Parkinson's disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol 58:158–167. https://doi.org/10.1002/jcph.1008
Park SH, Hwang MS, Park HJ, Shin HK, Baek JU, Choi BT (2018) Herbal prescriptions and medicinal herbs for parkinson-related rigidity in Korean medicine: identification of candidates using text mining. J Altern Complement Med 24:733–740. https://doi.org/10.1089/acm.2017.0387
Filograna R, Beltramini M, Bubacco L, Bisaglia M (2016) Anti-oxidants in Parkinson's disease therapy: a critical point of view. Curr Neuropharmacol 14:260–271. https://doi.org/10.2174/1570159X13666151030102718
Puspita L, Chung SY, Shim JW (2017) Oxidative stress and cellular pathologies in Parkinson's disease. Mol Brain 10:53. https://doi.org/10.1186/s13041-017-0340-9
Vaccari C, El Dib R, de Camargo JLV (2017) Paraquat and Parkinson's disease: a systematic review protocol according to the OHAT approach for hazard identification. Syst Rev 6:98. https://doi.org/10.1186/s13643-017-0491-x
Pouchieu C, Piel C, Carles C, Gruber A, Helmer C, Tual S, Marcotullio E, Lebailly P, Baldi I (2018) Pesticide use in agriculture and Parkinson's disease in the AGRICAN cohort study. Int J Epidemiol 47:299–310. https://doi.org/10.1093/ije/dyx225
Ortega-Arellano HF, Jimenez-Del-Rio M, Velez-Pardo C (2011) Life span and locomotor activity modification by glucose and polyphenols in Drosophila melanogaster chronically exposed to oxidative stress-stimuli: implications in Parkinson's disease. Neurochem Res 36:1073–1086. https://doi.org/10.1007/s11064-011-0451-0
Niveditha S, Ramesh SR, Shivanandappa T (2017) Paraquat-induced movement disorder in relation to oxidative stress-mediated neurodegeneration in the brain of Drosophila melanogaster. Neurochem Res 42:3310–3320. https://doi.org/10.1007/s11064-017-2373-y
Lavara-Culebras E, Muñoz-Soriano V, Gómez-Pastor R, Matallana E, Paricio N (2010) Effects of pharmacological agents on the lifespan phenotype of Drosophila DJ-1beta mutants. Gene 462:26–33. https://doi.org/10.1016/j.gene.2010.04.009
Navarro JA, Heßner S, Yenisetti SC, Bayersdorfer F, Zhang L, Voigt A, Schneuwly S, Botella JA (2014) Analysis of dopaminergic neuronal dysfunction in genetic and toxin-induced models of Parkinson's disease in Drosophila. J Neurochem 131:369–382. https://doi.org/10.1111/jnc.12818
Guo JD, Zhao X, Li Y, Li GR, Liu XL (2018) Damage to dopaminergic neurons by oxidative stress in Parkinson's disease (Review). Int J Mol Med 41:1817–1825. https://doi.org/10.3892/ijmm.2018.3406
Bonilla-Ramirez L, Jimenez-Del-Rio M, Velez-Pardo C (2013) Low doses of paraquat and polyphenols prolong life span and locomotor activity in knock-down parkin Drosophila melanogaster exposed to oxidative stress stimuli: implication in autosomal recessive juvenile parkinsonism. Gene 512:355–363. https://doi.org/10.1016/j.gene.2012.09.120
Ortega-Arellano HF, Jimenez-Del-Rio M, Velez-Pardo C (2017) Minocycline protects, rescues and prevents knockdown transgenic parkin Drosophila against paraquat/iron toxicity: implications for autosomic recessive juvenile parkinsonism. Neurotoxicology 60:42–53. https://doi.org/10.1016/j.neuro.2017.03.002
Maitra U, Ciesla L (2019) Using Drosophila as a platform for drug discovery from natural products in Parkinson’s disease. Med Chem Commun. https://doi.org/10.1039/C9MD00099B
Martinez-Perez DA, Jimenez-Del-Rio M, Velez-Pardo C (2018) Epigallocatechin-3-gallate protects and prevents paraquat-induced oxidative stress and neurodegeneration in knockdown dj-1-β Drosophila melanogaster. Neurotox Res 34:401–416. https://doi.org/10.1007/s12640-018-9899-x
Chen H, Morrell PL, Ashworth VETM, de la Cruz M, Clegg MT (2009) Tracing the geographic origins of major avocado cultivars. J Heredity 100:56–65. https://doi.org/10.1093/jhered/esn068
Bost JB, Smith NJH, Crane JH (2013) History, distribution and uses. In: Schaffer B, Nigel Wolstenholme B, Whiley AW (eds) The avocado: botany, production and uses, 2nd edn. CAB International, Boston, pp 10–30
Wang W, Bostic TR, Gu LW (2010) Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem 122:1193–1198. https://doi.org/10.1016/j.foodchem.2010.03.114
Ding H, Chin YW, Kinghorn AD, D'Ambrosio SM (2007) Chemopreventive characteristics of avocado fruit. Semin Cancer Biol 17:386–394. https://doi.org/10.1016/j.semcancer.2007.04.003
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 50:586–621. https://doi.org/10.1002/anie.201000044
Nabavi SF, Sureda A, Dehpour AR, Shirooie S, Silva AS, Devi KP, Ahmed T, Ishaq N, Hashim R, Sobarzo-Sánchez E, Daglia M, Braidy N, Volpicella M, Vacca RA, Nabavi SM (2017) Regulation of autophagy by polyphenols: paving the road for treatment of neurodegeneration. Biotechnol Adv 36:1768–1778. https://doi.org/10.1016/j.biotechadv.2017.12.001
Tremocoldi MA, Rosalen PL, Franchin M, Massarioli AP, Denny C, Daiuto ÉR, Paschoal JAR, Melo PS, Alencar SM (2018) Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 13:e0192577. https://doi.org/10.1371/journal.pone.0192577
Kujawska M, Jodynis-Liebert J (2018) Polyphenols in Parkinson’s disease: a systematic review of in vivo studies. Nutrients 10:E642. https://doi.org/10.3390/nu10050642
Rodríguez-Carpena JG, Morcuende D, Andrade MJ, Kylli P, Estévez M (2011) Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem 59:5625–5635. https://doi.org/10.1021/jf1048832
Kosińska A, Karamać M, Estrella I, Hernández T, Bartolomé B, Dykes GA (2012) Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J Agric Food Chem 9:4613–4619. https://doi.org/10.1021/jf300090p
Calderon-Oliver M, Escalona-Buendía HB, Medina-Campos ON, Pedraza-Chaverri J, Pedroza-Islas R, Ponce-Alquicira E (2016) Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT Food Sci Technol 65:46–52. https://doi.org/10.1016/j.lwt.2015.07.048
Nagoshi E (2011) Drosophila models of Sporadic Parkinson's disease. Int J Mol Sci 19(11):E3343. https://doi.org/10.3390/ijms19113343
Aryal B, Lee Y (2019) Disease model organism for Parkinson disease: Drosophila melanogaster. BMB Rep 52:250–258
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Álvarez R, Carvalho C, Sierra J, Lara O, Cardona D, Londono-Londoño J (2012) Citrus juice extraction systems: effect on chemical composition and antioxidant activity of clementine juice. J Agric Food Chem 60:774–781. https://doi.org/10.1021/jf203353h
Thaipong K, Boonprakoba U, Crosbyb K, Cisneros-Zevallosc L, Hawkins-Byrnec D (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Benzie IFF, Szeto YT (1999) Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J Agric Food Chem 47:633–636
Carrillo-Hormaza L, Ramírez AM, Osorio E, Gil A (2017) Optimization of ultrasound-assisted extraction and rapid resolution analysis of flavanols and methylxanthines for the quality control of cocoa-derived products. Food Anal Methods 10:497–507. https://doi.org/10.1007/s12161-016-0610-7
Crane JH, Douhan G, Faber BA, Arpaia ML, Bender GS, Balerdi CF, Barrientos-Priego AF (2013) Cultivars and rootstocks. In: Schaffer B, Nigel Wolstenholme B, Whiley AW (eds) The avocado: botany, production and uses, 2nd edn. CAB International, Boston, pp 200–233
Antasionasti I, Riyanto S, Rohman A (2017) Antioxidant activities and phenolics contents of avocado (Persea americana Mill.) peel in vitro. Res J Medic Plants 11:55–61. https://doi.org/10.3923/rjmp.2017.55.61
Smitha Grace SR, Chauhan JB, Jain CR (2015) Preliminary phytochemical investigation and tlc analysis of peel and seed extracts of Persea americana. Asian J Pharmac Sci Technol 5:167–171
Eghbaliferiz S, Iranshahi M (2016) Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytother Res 30:1379–1391. https://doi.org/10.1002/ptr.5643
Cherrak SA, Mokhtari-Soulimane N, Berroukeche F, Bensenane B, Cherbonnel A, Merzouk H, Elhabiri M (2016) In Vitro antioxidant versus metal ion chelating properties of flavonoids: a structure-activity investigation. PLoS ONE 11:e0165575. https://doi.org/10.1371/journal.pone.0165575
Zhang L, Wang Y, Li D, Ho CT, Li J, Wan X (2016) The absorption, distribution, metabolism and excretion of procyanidins. Food Funct 7:1273–1281. https://doi.org/10.1039/c5fo01244a
Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I (2018) Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem 241:480–492. https://doi.org/10.1016/j.foodchem.2017.08.117
Aaseth J, Dusek P, Roos PM (2018) Prevention of progression in Parkinson's disease. Biometals 31:737–747. https://doi.org/10.1007/s10534-018-0131-5
Ríos-Castaño D, Tafur Reyes RV (2003) Variedades de aguacate para el trópico: Caso Colombia. Proceedings V World Avocado Congress 2003 [Actas V Congreso Mundial del Aguacate 2003]. October 19 -24, 2003; Granada - Málaga, Spain, p. 143-147. (https://www.avocadosource.com/WAC5/WAC5_TOC.htm; available in April 2019).
Guzman LF, Machida-Hirano R, Borrayo E, Cortés-Cruz M, Espíndola-Barquera MD, Heredia-García E (2017) Genetic structure and selection of a core collection for long term conservation of avocado in Mexico. Front. Plant Sci 8:243. https://doi.org/10.3389/fpls.2017.00243
Hidalgo GI, Almajano MP (2017) Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: a review. Antioxidants (Basel) 6(1):E7. https://doi.org/10.3390/antiox6010007
Yuan Q, Zhao L (2017) The mulberry (Morus alba L.) fruit-A review of characteristic components and health benefits. J Agric Food Chem 65:10383–10394. https://doi.org/10.1021/acs.jafc.7b03614
Bar-Ya'akov I, Tian L, Amir R, Holland D (2019) Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front Plant Sci 10:620. https://doi.org/10.3389/fpls.2019.00620
Avila-Sosa R, Montero-Rodríguez AF, Aguilar-Alonso P, Vera-López O, Lazcano-Hernández M, Morales-Medina JC, Navarro-Cruz AR (2019) Antioxidant properties of amazonian fruits: a mini review of in vivo and in vitro studies. Oxid Med Cell Longev 2019:8204129. https://doi.org/10.1155/2019/8204129
Gu PS, Moon M, Choi JG, Oh MS (2017) Mulberry fruit ameliorates Parkinson's-disease-related pathology by reducing α-synuclein and ubiquitin levels in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model. J Nutr Biochem 39:15–21. https://doi.org/10.1016/j.jnutbio.2016.08.014
Mohammad-Beigi H, Aliakbari F, Sahin C, Lomax C, Tawfike A, Schafer NP, Amiri-Nowdijeh A, Eskandari H, Møller IM, Hosseini-Mazinani M, Christiansen G, Ward JL, Morshedi D, Otzen DE (2019) Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity. J Biol Chem 294:4215–4232. https://doi.org/10.1074/jbc.RA118.005723
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. https://doi.org/10.1038/nrdp.2017.13
Acknowledgements
The authors would like to thank the Ophidism and Scorpionism Research Group (Dr DM Benjumea-Gutierrez, JC Alarcon-Perez) and Bioactive Substances Service and Research Group (SIU-UdeA) for the use of the instruments and technical assistance. The authors would also like to thank the “Antioquean Avocado Corporation” (Corporacion Antioqueña del Aguacate, JC Ruiz-Perez) for the donation of plant material.
Funding
This work was supported by the “Committee for Development and Research” (Comité para el Desarrollo y la Investigación-CODI, Universidad de Antioquia-UdeA) Grants #2545. HFOA is a doctoral student from the Neuroscience program at the Basic Biomedical Sciences Academic Corporation-UdeA.
Author information
Authors and Affiliations
Contributions
MJ-Del-R and CV-P conceived and designed the experiments; HFO-A performed the experiments; HFO-A, CV-P, MJ-Del-R analyzed the data; MJ-Del-R contributed reagents/materials/analysis tools; CV-P, MJ-Del-R and HFO-A wrote and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
This study was carried out in accordance with National Legislation for Live Animal Experimentation (Colombia Republic, Resolution 08430, 1993). Experiments with Flies received the approval of the Ethics Committee for Animal Experimentation of the SIU-UdeA (act #83-2013).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ortega-Arellano, H.F., Jimenez-Del-Rio, M. & Velez-Pardo, C. Neuroprotective Effects of Methanolic Extract of Avocado Persea americana (var. Colinred) Peel on Paraquat-Induced Locomotor Impairment, Lipid Peroxidation and Shortage of Life Span in Transgenic knockdown Parkin Drosophila melanogaster. Neurochem Res 44, 1986–1998 (2019). https://doi.org/10.1007/s11064-019-02835-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02835-z