Abstract
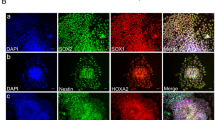
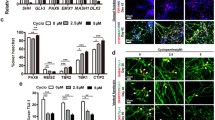
Recent advances in human induced pluripotent stem cells (hiPSCs) offer new possibilities for biomedical research and clinical applications. Neurons differentiated from hiPSCs may be promising tools to develop novel treatment methods for various neurological diseases. However, the detailed process underlying functional maturation of hiPSC-derived neurons remains poorly understood. Here, we analyze the developmental architecture of hiPSC-derived cortical neurons, iCell GlutaNeurons, focusing on the primary cilium, a single sensory organelle that protrudes from the surface of most growth-arrested vertebrate cells. To characterize the neuronal cilia, cells were cultured for various periods and evaluated immunohistochemically by co-staining with antibodies against ciliary markers Arl13b and MAP2. Primary cilia were detected in neurons within days, and their prevalence and length increased with increasing days in culture. Treatment with the mood stabilizer lithium led to primary cilia length elongation, while treatment with the orexigenic neuropeptide melanin-concentrating hormone caused cilia length shortening in iCell GlutaNeurons. The present findings suggest that iCell GlutaNeurons develop neuronal primary cilia together with the signaling machinery for regulation of cilia length. Our approach to the primary cilium as a cellular antenna can be useful for both assessment of neuronal maturation and validation of pharmaceutical agents in hiPSC-derived neurons.



Similar content being viewed by others
References
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME (2015) Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS ONE 10:e0118020. https://doi.org/10.1371/journal.pone.0118020
Ohara Y, Koganezawa N, Yamazaki H, Roppongi RT, Sato K, Sekino Y, Shirao T (2015) Early-stage development of human induced pluripotent stem cell-derived neurons. J Neurosci Res 93:1804–1813. https://doi.org/10.1002/jnr.23666
Nakamura H, Yamashita N, Kanamaru Y, Tachibana T, Sekino Y, Chen S, Gotoh T, Tanaka F, Goshima Y (2015) Quantitative analysis of intraneuronal transport in human iPS neurons. J Pharmacol Sci 128:170–178. https://doi.org/10.1016/j.jphs.2015.06.006
Dage JL, Colvin EM, Fouillet A et al (2014) Pharmacological characterisation of ligand- and voltage-gated ion channels expressed in human iPSC-derived forebrain neurons. Psychopharmacology 231:1105–1124. https://doi.org/10.1007/s00213-013-3384-2
Berry BJ, Akanda N, Smith AS, Long CJ, Schnepper MT, Guo X, Hickman JJ (2015) Morphological and functional characterization of human induced pluripotent stem cell-derived neurons (iCell Neurons) in defined culture systems. Biotechnol Prog 31:1613–1622. https://doi.org/10.1002/btpr.2160
Neagoe I, Liu C, Stumpf A, Lu Y, He D, Francis R, Chen J, Reynen P, Alaoui-Ismaili MH, Fukui H (2018) The GluN2B subunit represents a major functional determinant of NMDA receptors in human induced pluripotent stem cell-derived cortical neurons. Stem Cell Res 28:105–114. https://doi.org/10.1016/j.scr.2018.02.002
Louvi A, Grove EA (2011) Cilia in the CNS: the quiet organelle claims center stage. Neuron 69:1046–1060. https://doi.org/10.1016/j.neuron.2011.03.002
Ishikawa H, Marshall WF (2011) Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12:222–234. https://doi.org/10.1038/nrm3085
Wheway G, Nazlamova L, Hancock JT (2018) Signaling through the primary cilium. Front Cell Dev Biol 6:8. https://doi.org/10.3389/fcell.2018.00008
Hildebrandt F, Benzing T, Katsanis N (2011) Ciliopathies. New Engl J Med 364:1533–1543. https://doi.org/10.1056/NEJMra1010172
Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, Sarkisian MR (2013) Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci 33:2626–2638. https://doi.org/10.1523/JNEUROSCI.2906-12.2013
Guo J, Otis JM, Higginbotham H, Monckton C, Cheng J, Asokan A, Mykytyn K, Caspary T, Stuber GD, Anton ES (2017) Primary cilia signaling shapes the development of interneuronal connectivity. Dev Cell 42:286–300. https://doi.org/10.1016/j.devcel.2017.07.010
Hilgendorf KI, Johnson CT, Jackson PK (2016) The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol 39:84–92. https://doi.org/10.1016/j.ceb.2016.02.008
Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K (2008) Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA 105:4242–4246. https://doi.org/10.1073/pnas.0711027105
Miyoshi K, Kasahara K, Miyazaki I, Asanuma M (2009) Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun 388:757–762. https://doi.org/10.1016/j.bbrc.2009.08.099
Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K (2011) Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci 68:2951–2960. https://doi.org/10.1007/s00018-010-0603-4
Kobayashi Y, Takemoto R, Yamato S, Okada T, Iijima M, Uematsu Y, Chaki S, Saito Y (2018) Depression-resistant phenotype in mice overexpressing regulator of G protein signaling 8 (RGS8). Neuroscience 383:160–169. https://doi.org/10.1016/j.neuroscience.2018.05.005
Marley A, von Zastrow M (2012) A simple cell-based assay reveals that diverse neuropsychiatric risk genes converge on primary cilia. PLoS ONE 7:e46647. https://doi.org/10.1371/journal.pone.0046647
Hu L, Wang B, Zhang Y (2017) Serotonin 5-HT6 receptors affect cognition in a mouse model of Alzheimer’s disease by regulating cilia function. Alzheimers Res Ther 9:76. https://doi.org/10.1186/s13195-017-0304-4
Liang Y, Meng D, Zhu B, Pan J (2016) Mechanism of ciliary disassembly. Cell Mol Life Sci 73:1787–1802. https://doi.org/10.1007/s00018-016-2148-7
Hamamoto A, Yamato S, Katoh Y, Nakayama K, Yoshimura K, Takeda S, Kobayashi Y, Saito Y (2016) Modulation of primary cilia length by melanin-concentrating hormone receptor 1. Cell Signal 28:572–584. https://doi.org/10.1016/j.cellsig.2016.02.018
Tomoshige S, Kobayashi Y, Hosoba K, Hamamoto A, Miyamoto T, Saito Y (2017) Cytoskeleton-related regulation of primary cilia shortening mediated by melanin-concentrating hormone receptor 1. Gen Comp Endocrinol 253:44–52. https://doi.org/10.1016/j.ygcen.2017.08.021
Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37:173–182. https://doi.org/10.1016/0165-0270(91)90128-M
Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES (2012) Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 23:925–938. https://doi.org/10.1016/j.devcel.2012.09.019
Lee JY, Stearns T (2013) FOP is a centriolar satellite protein involved in ciliogenesis. PLoS ONE 8:e58589. https://doi.org/10.1371/journal.pone.0058589
Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA (2009) Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res 315:2802–2817. https://doi.org/10.1016/j.yexcr.2009.06.028
Muñoz-Estrada J, Lora-Castellanos A, Meza I, Alarcón Elizalde S, Benítez-King G (2018) Primary cilia formation is diminished in schizophrenia and bipolar disorder: a possible marker for these psychiatric diseases. Schizophr Res 195:412–420. https://doi.org/10.1016/j.schres.2017.08.055
May-Simera HL, Wan Q, Jha BS et al (2018) Primary cilium-mediated retinal pigment epithelium maturation is disrupted in ciliopathy patient cells. Cell Rep 22:189–205. https://doi.org/10.1016/j.celrep.2017.12.038
Sterpka A, Chen X (2018) Neuronal and astrocytic primary cilia in the mature brain. Pharmacol Res 137:114–121. https://doi.org/10.1016/j.phrs.2018.10.002
Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR (2012) Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol 520:848–873. https://doi.org/10.1002/cne.22793
Chambers J, Ames RS, Bergsma D et al (1999) Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature 400:261–265. https://doi.org/10.1038/22313
Saito Y, Nothacker HP, Wang Z, Lin S, Leslie FM, Civelli O (1999) Molecular characterization of the melanin-concentrating-hormone receptor. Nature 400:265–269. https://doi.org/10.1038/22321
Macneil DJ (2013) The role of melanin-concentrating hormone and its receptors in energy homeostasis. Front Endocrinol 4:49. https://doi.org/10.3389/fendo.2013.00049
Barnett S, Reilly S, Carr L, Ojo I, Beales PL, Charman T (2002) Behavioural phenotype of Bardet-Biedl syndrome. J Med Genet 39:e76. https://doi.org/10.1136/jmg.39.12.e76
Acknowledgements
The study was supported by research grants (AMED JP17bk0104077 to T.S.) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI 16K07053 to N.T and 15K06775 to Y.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miki, D., Kobayashi, Y., Okada, T. et al. Characterization of Functional Primary Cilia in Human Induced Pluripotent Stem Cell-Derived Neurons. Neurochem Res 44, 1736–1744 (2019). https://doi.org/10.1007/s11064-019-02806-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02806-4