Abstract
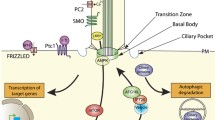
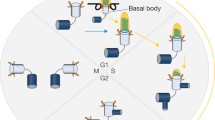
As motile organelles and sensors, cilia play pivotal roles in cell physiology, development and organ homeostasis. Ciliary defects are associated with a class of cilia-related diseases or developmental disorders, termed ciliopathies. Even though the presence of cilia is required for diverse functions, cilia can be removed through ciliary shortening or resorption that necessitates disassembly of the cilium, which occurs normally during cell cycle progression, cell differentiation and in response to cellular stress. The functional significance of ciliary resorption is highlighted in controlling the G1-S transition during cell cycle progression. Internal or external cues that trigger ciliary resorption initiate signaling cascades that regulate several downstream events including depolymerization of axonemal microtubules, dynamic changes in actin and the ciliary membrane, regulation of intraflagellar transport and posttranslational modifications of ciliary proteins. To ensure ciliary resorption, both the active disassembly of the cilium and the simultaneous inhibition of ciliary assembly must be coordinately regulated.





Similar content being viewed by others
Abbreviations
- IFT:
-
Intraflagellar transport
- MT:
-
Microtubule
- NaPPi:
-
Sodium pyrophosphate
- Plk1:
-
Polo-like kinase 1
- Dvl2:
-
Dishevelled 2
References
Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22:541–546
Nachury MV, Seeley ES, Jin H (2010) Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26:59–87
Satir P, Christensen ST (2007) Overview of structure and function of mammalian cilia. Annu Rev Physiol 69:377–400
Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11:331–344
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19:R526–R535
Brown JM, Witman GB (2014) Cilia and diseases. Bioscience 64:1126–1137
Reiter JF, Blacque OE, Leroux MR (2012) The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 13:608–618
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3:813–825
Ounjai P, Kim KD, Liu H, Dong M, Tauscher AN, Witkowska HE, Downing KH (2013) Architectural insights into a ciliary partition. Curr Biol 23:339–344
Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, Verhey KJ (2012) A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol 14:431–437
Scholey JM (2003) Intraflagellar transport. Annu Rev Cell Dev Biol 19:423–443
Marshall WF, Rosenbaum JL (2001) Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol 155:405–414
Jackson PK (2011) Do cilia put brakes on the cell cycle? Nat Cell Biol 13:340–342
Pan J, Snell W (2007) The primary cilium: keeper of the key to cell division. Cell 129:1255–1257
Bloodgood RA (1974) Resorption of organelles containing microtubules. Cytobios 9:142–161
Sanders MA, Salisbury JL (1989) Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol 108:1751–1760
Blum JJ (1971) Existence of a breaking point in cilia and flagella. J Theor Biol 33:257–263
Rosenbaum JL, Child FM (1967) Flagellar regeneration in protozoan flagellates. J Cell Biol 34:345–364
Randall SJ, Cavalier-Smith T, McVittie A, Warr JR, Hopkins JM (1967) Developmental and control processes in the basal bodies and flagella of Chlamydomonas reinhardtii. Dev Biol Suppl 1:43–83
Goto H, Inoko A, Inagaki M (2013) Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci 70:3893–3905
Kim S, Tsiokas L (2011) Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle 10:2683–2690
Pan J, Seeger-Nukpezah T, Golemis EA (2013) The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies. Cell Mol Life Sci 70:1849–1874
Cao M, Li G, Pan J (2009) Regulation of cilia assembly, disassembly, and length by protein phosphorylation. Methods Cell Biol 94:333–346
Lefebvre PA, Rosenbaum JL (1986) Regulation of the synthesis and assembly of ciliary and flagellar proteins during regeneration. Annu Rev Cell Biol 2:517–546
Seeger-Nukpezah T, Little JL, Serzhanova V, Golemis EA (2013) Cilia and cilia-associated proteins in cancer. Drug Discov Today Dis Mech 10:e135–e142
Sung CH, Li A (2011) Ciliary resorption modulates G1 length and cell cycle progression. Cell Cycle 10:2825–2826
Prodromou NV, Thompson CL, Osborn DP, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP (2012) Heat shock induces rapid resorption of primary cilia. J Cell Sci 125:4297–4305
McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA (2010) Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int 34:441–446
Iomini C, Tejada K, Mo W, Vaananen H, Piperno G (2004) Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 164:811–817
Luo N, Conwell MD, Chen X, Kettenhofen CI, Westlake CJ, Cantor LB, Wells CD, Weinreb RN, Corson TW, Spandau DF et al (2014) Primary cilia signaling mediates intraocular pressure sensation. Proc Natl Acad Sci USA 111:12871–12876
Solter KM, Gibor A (1978) The relationship between tonicity and flagellar length. Nature 275:651–652
Telser A (1977) The inhibition of flagellar regeneration in Chlamydomonas reinhardii by inhalational anesthetic halothane. Exp Cell Res 107:247–252
Lefebvre PA, Nordstrom SA, Moulder JE, Rosenbaum JL (1978) Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol 78:8–27
Rosenbaum JL, Moulder JE, Ringo DL (1969) Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol 41:600–619
Rannestad J (1974) The regeneration of cilia in partially deciliated Tetrahymena. J Cell Biol 63:1009–1017
Kikuchi K, Hilding D (1965) The development of the organ of Corti in the mouse. Acta Otolaryngol 60:207–222
Kimura RS (1966) Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol 61:55–72
Rozycki M, Lodyga M, Lam J, Miranda MZ, Fatyol K, Speight P, Kapus A (2014) The fate of the primary cilium during myofibroblast transition. Mol Biol Cell 25:643–657
Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST (2005) PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15:1861–1866
Goetz SC, Ocbina PJ, Anderson KV (2009) The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol 94:199–222
Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P (2009) Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells 27:703–713
Forcioli-Conti N, Lacas-Gervais S, Dani C, Peraldi P (2015) The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells. Biochem Biophys Res Commun 458:117–122
Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H (2009) Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci USA 106:1820–1825
Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci 16:529–556
Pan J, Snell WJ (2005) Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev Cell 9:431–438
Zilberstein D, Shapira M (1994) The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 48:449–470
Wheatley DN, Wang AM, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73–81
Dingemans KP (1969) The relation between cilia and mitoses in the mouse adenohypophysis. J Cell Biol 43:361–367
Rash JE, Shay JW, Biesele JJ (1969) Cilia in cardiac differentiation. J Ultrastruct Res 29:470–484
Fonte VG, Searls RL, Hilfer SR (1971) The relationship of cilia with cell division and differentiation. J Cell Biol 49:226–229
Tucker RW, Pardee AB, Fujiwara K (1979) Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell 17:527–535
Archer FL, Wheatley DN (1971) Cilia in cell-cultured fibroblasts. II. Incidence in mitotic and post-mitotic BHK 21-C13 fibroblasts. J Anat 109:277–292
Spalluto C, Wilson DI, Hearn T (2013) Evidence for reciliation of RPE1 cells in late G1 phase, and ciliary localisation of cyclin B1. FEBS Open Bio 3:334–340
Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129:1351–1363
Rieder CL, Jensen CG, Jensen LC (1979) The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res 68:173–185
Tucker RW, Scher CD, Stiles CD (1979) Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell 18:1065–1072
Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH (2011) Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol 13:402–411
Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L (2011) Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol 13:351–360
Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, Enomoto M, Ibi M, Urano T, Yonemura S, Kiyono T et al (2012) Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol 197:391–405
Hu Z, Liang Y, He W, Pan J (2015) Cilia disassembly with two distinct phases of regulation. Cell Rep 10:1803–1810
Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL (2005) Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell 16:270–278
Langousis G, Hill KL (2014) Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol 12:505–518
Friedlander M, Wahrman J (1970) The spindle as a basal body distributor. A study in the meiosis of the male silkworm moth, Bombyx mori. J Cell Sci 7:65–89
Riparbelli MG, Callaini G, Megraw TL (2012) Assembly and persistence of primary cilia in dividing Drosophila spermatocytes. Dev Cell 23:425–432
Pazour GJ, Agrin N, Leszyk J, Witman GB (2005) Proteomic analysis of a eukaryotic cilium. J Cell Biol 170:103–113
L’Hernault SW, Rosenbaum JL (1983) Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol 97:258–263
L’Hernault SW, Rosenbaum JL (1985) Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24:473–478
Wloga D, Gaertig J (2010) Post-translational modifications of microtubules. J Cell Sci 123:3447–3455
Janke C, Bulinski JC (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12:773–786
Piperno G, Fuller MT (1985) Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101:2085–2094
Song Y, Brady ST (2015) Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol 25:125–136
Vashishtha M, Walther Z, Hall JL (1996) The kinesin-homologous protein encoded by the Chlamydomonas FLA10 gene is associated with basal bodies and centrioles. J Cell Sci 109(Pt 3):541–549
Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A (2014) Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157:1405–1415
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J (2010) MEC-17 is an alpha-tubulin acetyltransferase. Nature 467:218–222
Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV (2010) The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA 107:21517–21522
Kozminski KG, Diener DR, Rosenbaum JL (1993) High level expression of nonacetylatable alpha-tubulin in Chlamydomonas reinhardtii. Cell Motil Cytoskeleton 25:158–170
Ran J, Yang Y, Li D, Liu M, Zhou J (2015) Deacetylation of alpha-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci Rep 5:12917
Walczak CE, Gayek S, Ohi R (2013) Microtubule-depolymerizing kinesins. Annu Rev Cell Dev Biol 29:417–441
Vasudevan KK, Jiang YY, Lechtreck KF, Kushida Y, Alford LM, Sale WS, Hennessey T, Gaertig J (2015) Kinesin-13 regulates the quantity and quality of tubulin inside cilia. Mol Biol Cell 26:478–494
Hu Z, Liang Y, Meng D, Wang L, Pan J (2015) Microtubule-depolymerizing kinesins in the regulation of assembly, disassembly, and length of cilia and flagella. Int Rev Cell Mol Biol 317:241–265
Niwa S, Nakajima K, Miki H, Minato Y, Wang D, Hirokawa N (2012) KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev Cell 23:1167–1175
Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ (2007) Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in giardia intestinalis. Eukaryot Cell 6:2354–2364
Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P (2007) A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol 17:778–782
Chan KY, Ersfeld K (2010) The role of the Kinesin-13 family protein TbKif13-2 in flagellar length control of Trypanosoma brucei. Mol Biochem Parasitol 174:137–140
Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J (2009) A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA 106:4713–4718
Miyamoto T, Hosoba K, Ochiai H, Royba E, Izumi H, Sakuma T, Yamamoto T, Dynlacht BD, Matsuura S (2015) The microtubule-depolymerizing activity of a mitotic kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Rep 10:664–673
Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL (2004) Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol 164:255–266
Werner-Peterson R, Sloboda RD (2013) Methylation of structural components of the axoneme occurs during flagellar disassembly. Biochemistry 52:8501–8509
Sloboda RD, Howard L (2009) Protein methylation in full length Chlamydomonas flagella. Cell Motil Cytoskeleton 66:650–660
Schneider MJ, Ulland M, Sloboda RD (2008) A protein methylation pathway in Chlamydomonas flagella is active during flagellar resorption. Mol Biol Cell 19:4319–4327
Hochstrasser M (1996) Ubiquitin-dependent protein degradation. Annu Rev Genet 30:405–439
Kirkin V, Dikic I (2007) Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr Opin Cell Biol 19:199–205
Huang K, Diener DR, Rosenbaum JL (2009) The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol 186:601–613
Inglis PN, Boroevich KA, Leroux MR (2006) Piecing together a ciliome. Trends Genet 22:491–500
Wang W, Wu T, Kirschner MW (2014) The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. Elife 3:e03083
Sun L, Chen ZJ (2004) The novel functions of ubiquitination in signaling. Curr Opin Cell Biol 16:119–126
Pan J, Wang Q, Snell WJ (2004) An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell 6:445–451
Pan J, Naumann-Busch B, Wang L, Specht M, Scholz M, Trompelt K, Hippler M (2011) Protein phosphorylation is a key event of flagellar disassembly revealed by analysis of flagellar phosphoproteins during flagellar shortening in Chlamydomonas. J Proteome Res 10:3830–3839
Brown DL, Rogers KA (1978) Hydrostatic pressure-induced internalization of flagellar axonemes, disassembly, and reutilization during flagellar regeneration in Polytomella. Exp Cell Res 117:313–324
Liang Y, Pang Y, Wu Q, Hu Z, Han X, Xu Y, Deng H, Pan J (2014) FLA8/KIF3B phosphorylation regulates kinesin-II interaction with IFT-B to control IFT entry and turnaround. Dev Cell 30:585–597
Mc LR, Laurendi CJ, Brown RM Jr (1974) The relationship of gamone to the mating reaction in Chlamydomonas moewusii. Proc Natl Acad Sci USA 71:2610–2613
Wood CR, Rosenbaum JL (2015) Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol 25:276–285
Wood CR, Rosenbaum JL (2014) Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr Biol 24:1114–1120
Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM (2014) C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24:519–525
Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ et al (2009) Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20:278–288
Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M (2007) Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol 176:483–495
Yan X, Zhu X (2013) Branched F-actin as a negative regulator of cilia formation. Exp Cell Res 319:147–151
Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, Zhu X (2012) miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol 14:697–706
Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee JK, Heo WD, Kim J (2015) Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun 6:6781
Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19:270–283
Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464:1048–1051
Lua BL, Low BC (2005) Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett 579:577–585
Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR et al (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 27:197–213
Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH (2005) The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell 9:75–86
Avasthi P, Onishi M, Karpiak J, Yamamoto R, Mackinder L, Jonikas MC, Sale WS, Shoichet B, Pringle JR, Marshall WF (2014) Actin is required for IFT regulation in Chlamydomonas reinhardtii. Curr Biol 24:2025–2032
Dentler WL, Adams C (1992) Flagellar microtubule dynamics in Chlamydomonas: cytochalasin D induces periods of microtubule shortening and elongation; and colchicine induces disassembly of the distal, but not proximal, half of the flagellum. J Cell Biol 117:1289–1298
Kato-Minoura T, Uryu S, Hirono M, Kamiya R (1998) Highly divergent actin expressed in a Chlamydomonas mutant lacking the conventional actin gene. Biochem Biophys Res Commun 251:71–76
Hirono M, Uryu S, Ohara A, Kato-Minoura T, Kamiya R (2003) Expression of conventional and unconventional actins in Chlamydomonas reinhardtii upon deflagellation and sexual adhesion. Eukaryot Cell 2:486–493
Kim S, Lee K, Choi JH, Ringstad N, Dynlacht BD (2015) Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun 6:8087
Huang B, Rifkin MR, Luck DJ (1977) Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol 72:67–85
Adams GM, Huang B, Luck DJ (1982) Temperature-sensitive, assembly-defective flagella mutants of Chlamydomonas reinhardtii. Genetics 100:579–586
Miller MS, Esparza JM, Lippa AM, Lux FG, Cole DG, Dutcher SK (2005) Mutant kinesin-2 motor subunits increase chromosome loss. Mol Biol Cell 16:3810–3820
Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90:5519–5523
Liang Y, Pan J (2013) Regulation of flagellar biogenesis by a calcium dependent protein kinase in Chlamydomonas reinhardtii. PLoS One 8:e69902
Cheshire JL, Evans JH, Keller LR (1994) Ca2+ signaling in the Chlamydomonas flagellar regeneration system: cellular and molecular responses. J Cell Sci 107(Pt 9):2491–2498
Cheshire JL, Keller LR (1991) Uncoupling of Chlamydomonas flagellar gene expression and outgrowth from flagellar excision by manipulation of Ca2+. J Cell Biol 115:1651–1659
Quader H, Cherniack J, Filner P (1978) Participation of calcium in flagellar shortening and regeneration in Chlamydomonas reinhardii. Exp Cell Res 113:295–301
Tucker RW, Meade-Cobun KS, Jayaraman S, More NS (1983) Centrioles, primary cilia and calcium in the growth of Balb/c 3T3 cells. J Submicrosc Cytol 15:139–143
Plotnikova OV, Pugacheva EN, Dunbrack RL, Golemis EA (2010) Rapid calcium-dependent activation of Aurora-A kinase. Nat Commun 1:64
Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV (2010) Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20:182–187
Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA (2012) Calmodulin activation of Aurora-A kinase (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell 23:2658–2670
Tuxhorn J, Daise T, Dentler WL (1998) Regulation of flagellar length in Chlamydomonas. Cell Motil Cytoskeleton 40:133–146
Lefebvre PA, Silflow CD, Wieben ED, Rosenbaum JL (1980) Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell 20:469–477
Pasquale SM, Goodenough UW (1987) Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J Cell Biol 105:2279–2292
Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA (2009) Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res 315:2802–2817
Abdul-Majeed S, Moloney BC, Nauli SM (2012) Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci 69:165–173
Pan J, Snell WJ (2000) Regulated targeting of a protein kinase into an intact flagellum. An aurora/Ipl1p-like protein kinase translocates from the cell body into the flagella during gamete activation in Chlamydomonas. J Biol Chem 275:24106–24114
Cao M, Meng D, Wang L, Bei S, Snell WJ, Pan J (2013) Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length. Proc Natl Acad Sci USA 110:12337–12342
Bhogaraju S, Weber K, Engel BD, Lechtreck KF, Lorentzen E (2014) Getting tubulin to the tip of the cilium: one IFT train, many different tubulin cargo-binding sites? BioEssays 36:463–467
Pan J, Snell WJ (2014) Organelle size: a cilium length signal regulates IFT cargo loading. Curr Biol 24:R75–R78
Wang L, Piao T, Cao M, Qin T, Huang L, Deng H, Mao T, Pan J (2013) Flagellar regeneration requires cytoplasmic microtubule depolymerization and kinesin-13. J Cell Sci 126:1531–1540
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S et al (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831
Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, Bang JK, Zhou M, Veenstra TD, Yeon Kim B, Lee KS (2012) Identification of a novel Wnt5a-CK1varepsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J 31:3104–3117
Kinzel D, Boldt K, Davis EE, Burtscher I, Trumbach D, Diplas B, Attie-Bitach T, Wurst W, Katsanis N, Ueffing M et al (2010) Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell 19:66–77
Lienkamp S, Ganner A, Walz G (2012) Inversin, Wnt signaling and primary cilia. Differentiation 83:S49–S55
Wallingford JB, Mitchell B (2011) Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25:201–213
Wang G, Chen Q, Zhang X, Zhang B, Zhuo X, Liu J, Jiang Q, Zhang C (2013) PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry. J Cell Sci 126:1355–1365
Yeh C, Li A, Chuang JZ, Saito M, Caceres A, Sung CH (2013) IGF-1 activates a cilium-localized noncanonical Gbetagamma signaling pathway that regulates cell-cycle progression. Dev Cell 26:358–368
Hou Y, Witman GB (2015) Dynein and intraflagellar transport. Exp Cell Res 334:26–34
Stehman SA, Chen Y, McKenney RJ, Vallee RB (2007) NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol 178:583–594
Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM (2013) The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr Biol 23:2208–2214
Parker JD, Quarmby LM (2003) Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biol 4:1471–2121
Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M, Gaertig J (2006) Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell 17:2799–2810
Berman SA, Wilson NF, Haas NA, Lefebvre PA (2003) A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol 13:1145–1149
Tam LW, Ranum PT, Lefebvre PA (2013) CDKL5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas. Mol Biol Cell 24:588–600
Tam LW, Wilson NF, Lefebvre PA (2007) A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol 176:819–829
Acknowledgments
We thank Drs. Roger Sloboda and Kaiyao Huang for critical review and editing of this manuscript. This work was supported by awards from the National Basic Research Program of China (2012CB945000 and 2013CB910700) and the National Science Foundation of China (31330044) to J. P. Y. L is supported by a Postdoctoral Fellowship from Tsinghua-Peking Center for Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Y., Meng, D., Zhu, B. et al. Mechanism of ciliary disassembly. Cell. Mol. Life Sci. 73, 1787–1802 (2016). https://doi.org/10.1007/s00018-016-2148-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2148-7