Abstract
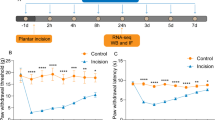
Neuropathic pain is an intractable disease with few definitive therapeutic options. Anethole (AN) has been confirmed to possess potent anti-inflammatory and neuroprotective properties, but its effect on neuropathic pain has not been reported. The present study was designed to investigate the antinociceptive effect of AN on chronic constriction injury (CCI)-induced neuropathic pain in mice. AN (125, 250, and 500 mg/kg) and pregabalin (40 mg/kg) were intragastric administered for 8 consecutive days from the 7th day post-surgery. Behavioral parameters were measured on different days, namely, 0, 7, 8, 10, 12, and 14, from CCI operation. Additionally, electrophysiological and histopathological changes were analyzed on the 14th day. Afterward, immunofluorescence and Western blot were utilized to examine the activation of glial cells and the expression of inflammatory cytokines, respectively. AN treatment of CCI mice considerably alleviated hyperalgesia and allodynia, ameliorated abnormal sciatic nerve conduction, and restored injured sciatic nerves in a dose-dependent manner. Furthermore, AN suppressed the activation of glial cells, down-regulated pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL-6, and IL-1β), and up-regulated the anti-inflammatory cytokine (IL-10). These assays first indicated that AN exerted an antinociceptive effect on CCI-induced neuropathic pain, and might be attributed to the anti-inflammatory and neuroprotective activities of AN.











Similar content being viewed by others
References
Haanpää M (2010) NeuPSIG guidelines on neuropathic pain assessment. Eur J Neurol 17(8):1010
Kerstman E, Ahn S, Battu S, Tariq S, Grabois M (2013) Chap. 15—Neuropathic pain. Elsevier Health Sciences, Philadelphia, pp 175–187
DiBonaventura MD, Sadosky A, Concialdi K, Hopps M, Kudel I, Parsons B, Cappelleri JC, Hlavacek P, Alexander AH, Stacey BR, Markman JD, Farrar JT (2017) The prevalence of probable neuropathic pain in the US: results from a multimodal general-population health survey. J Pain Res 10:2525–2538
O’Connor AB (2009) Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics 27(2):95–112
Gong SS, Li YX, Zhang MT, Du J, Ma PS, Yao WX, Zhou R, Niu Y, Sun T, Yu JQ (2016) Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem Res 41(11):1–13
Jie W, Jones M, Tanaka M, Selvaraj P, Symes AJ, Cox B, Zhang Y (2018) WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms. J Neuroinflamm 15(1):9
Lee JY, Choi HY, Ju BG, Yune TY (2018) Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim Biophys Acta 1864:2472–2480
Zahner G, Schaper M, Panzer U, Kluger M, Stahl RA, Thaiss F, Schneider A (2009) Prostaglandin EP2 and EP4 receptors modulate expression of the chemokine CCL2 (MCP-1) in response to LPS-induced renal glomerular inflammation. Biochem J 422(3):563–570
Montague K, Simeoli R, Valente J, Malcangio M (2018) A novel interaction between CX3CR1 and CCR2 signalling in monocytes constitutes an underlying mechanism for persistent vincristine-induced pain. J Neuroinflamm 15(1):101
Tsuda M, Inoue K (2016) Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology 104:76–81
Yang Y, Hu L, Xia Y, Jiang C, Miao C, Yang C, Yuan M, Wang L (2016) Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflamm 13(1):84
Bridges D, Thompson SW, Rice AS (2001) Mechanisms of neuropathic pain. Br J Anaesth 87(1):12–26
Gao YJ, Ji RR (2010) Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol Ther 126(1):56–68
Milligan E, Watkins L (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10(1):23
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10(11):1361
Tao L, Ding Q, Gao C, Sun X (2016) Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int Immunopharmacol 34:165–172
Eliav E, Benoliel R, Herzberg U, Kalladka M, Tal M (2009) The role of IL-6 and IL-1beta in painful perineural inflammatory neuritis. Brain Behav Immun 23(4):474–484
Sommer C (1999) Animal studies on neuropathic pain: the role of cytokines and cytokine receptors in pathogenesis and therapy. Schmerz 13(5):315–323
Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21(5):522–527
Milligan ED, Langer SJ, Sloane EM, He L, Wieselerfrank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K (2015) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21(8):2136–2148
Wiffen PJ, Mcquay HJ, Edwards J, Moore RA (2011) Gabapentin for acute and chronic pain. Cochrane Database Syst Rev 3(2):CD006044
Zareba G (2009) Phytotherapy for pain relief. Drugs Today 45(6):445–467
Modaress NV, Asadipour M (2006) Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. East Mediterr Health J 12 (3–4):423
Javidnia K, Dastgheib L, Mohammadi SS, Nasiri A (2003) Antihirsutism activity of fennel (fruits of Foeniculum vulgare) extract. A double-blind placebo controlled study. Phytomedicine 10(6):455–458
Choi EM, Hwang JK (2004) Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 75(6):557–565
Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB (2000) Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene 19(25):2943–2950
Kang P, Kim KY, Lee HS, Min SS, Seol GH (2013) Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci 93(24):955–961
Geronikaki AA, Gavalas AM (2006) Antioxidants and inflammatory disease: synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen 9(6):425–442
Newberne P, Smith RL, Doull J, Goodman JI, Munro IC, Portoghese PS, Wagner BM, Weil CS, Woods LA, Adams TB (1999) The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Flavour and Extract Manufacturer’s Association. Food Chem Toxicol 37(7):789–811
Abraham SK (2001) Anti-genotoxicity of trans-anethole and eugenol in mice. Food Chem Toxicol 39(5):493–498
Ritter AM, Domiciano TP Jr, Zarpelon VW, Da AC, Barbosa SL, Natali CP, Cuman MR, Bersani-Amado RK CA (2013) Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 21(2):187–197
Aprotosoaie AC, Costache II, Miron A (2016) Anethole and its role in chronic diseases. Oxyg Transp Tissue XXXIII 929:247–267
Drukarch B, Schepens E, Stoof JC, Langeveld CH (1997) Anethole dithiolethione prevents oxidative damage in glutathione-depleted astrocytes. Eur J Pharmacol 329(2–3):259
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33(1):87
Hervera A, Negrete R, Leánez S, Martín-Campos J, Pol O (2010) The role of nitric oxide in the local antiallodynic and antihyperalgesic effects and expression of delta-opioid and cannabinoid-2 receptors during neuropathic pain in mice. J Pharmacol Exp Ther 334(3):887
Darwish IS, Dessouky IS (2015) Does serum Visfatin represent a biochemical marker to an experimental peripheral neuropathic pain in mice. Pharmacology 96(5–6):248–252
Domiciano TP, Dalalio MM, Silva EL, Ritter AM, Estevão-Silva CF, Ramos FS, Caparroz-Assef SM, Cuman RK, Bersani-Amado CA (2013) Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn-Schmiedeberg’s Arch Pharmacol 386(4):331–338
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Jasmin L, Kohan L, Franssen M, Janni G, Goff JR (1998) The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain 75(2–3):367
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (2015) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32(1):77–88
Beyreuther BK, Geis C, Stöhr T, Sommer C (2007) Antihyperalgesic efficacy of lacosamide in a rat model for muscle pain induced by TNF. Neuropharmacology 52(5):1312–1317
Kayser V, Farré A, Hamon M, Bourgoin S (2003) Effects of the novel analgesic, cizolirtine, in a rat model of neuropathic pain. Pain 104(1–2):169–177
Ja’Afer FM, Hamdan FB, Mohammed FH (2006) Vincristine-induced neuropathy in rat: electrophysiological and histological study. Exp Brain Res 173(2):334–345
Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL (2012) Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 83(4):650–659
Liu N, Li YX, Gong SS, Du J, Liu G, Jin SJ, Zhao CJ, Niu Y, Sun T, Yu JQ (2016) Antinociceptive effects of gentiopicroside on neuropathic pain induced by chronic constriction injury in mice: a behavioral and electrophysiological study. Can J Physiol Pharmacol 94(7):1–10
Gould HJ, Soignier RD, Cho SR, Hernandez C, Diamond I, Taylor BK, Paul D (2014) Ranolazine attenuates mechanical allodynia associated with demyelination injury. Pain Med 15(10):1771
Sudoh Y, Desai SP, Haderer AE, Sudoh S, Gerner P, Anthony DC, De GU, Wang GK (2004) Neurologic and histopathologic evaluation after high-volume intrathecal amitriptyline. Reg Anesth Pain Med 29(5):434–440
Xu L, Zhou S, Feng GY, Zhang LP, Zhao DM, Sun Y, Liu Q, Huang F (2012) Neural stem cells enhance nerve regeneration after sciatic nerve injury in rats. Mol Neurobiol 46(2):265–274
Woolf CJ, Mannion RJ (1999) Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353(9168):1959–1964
Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J, Tian Y, Mao-Ying QL, Jiang JW, Ma HJ (2014) Aspirin-triggered Lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience 273:65–78
Wang B, Liu S, Fan B, Xu X, Chen Y, Lu R, Xu Z, Liu X (2018) PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J Headache Pain 19(1):7
Yeesuk K, Huejung P, Taekwan K, Dongeon M, Haejin L (2009) The effects of Ginkgo biloba extract EGb 761 on mechanical and cold allodynia in a rat model of neuropathic pain. Anesth Analgesia 108(6):1958–1963
Zhang MT, Wang B, Jia YN, Liu N, Ma PS, Gong SS, Niu Y, Sun T, Li YX, Yu JQ (2017) Neuroprotective effect of liquiritin against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Biomed Pharmacother 95(1):186–198
Uçeyler N, Kobsar I, Biko L, Ulzheimer J, Levinson SR, Martini R, Sommer C (2006) Heterozygous P0 deficiency protects mice from vincristine-induced polyneuropathy. J Neurosci Res 84(1):37–46
Lehning EJ, Jortner BS, Fox JH, Arezzo JC, Kitano T, Lopachin RM (2000) gamma-diketone peripheral neuropathy. I. Quality morphometric analyses of axonal atrophy and swelling. Toxicol Appl Pharmacol 165(2):127–140
Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy-Pour A (2014) Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience 256(1):403–411
Popiolek-Barczyk K, Mika J (2016) Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem 23(26):2908–2928
Rojewska E, Piotrowska A, Popiolekbarczyk K, Mika J (2018) Botulinum toxin type A-A modulator of spinal neuron-glia interactions under neuropathic pain conditions. Toxins 10(4):145
Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51(2):240–264
Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ (2008) Nociceptors are interleukin-1beta sensors. J Neurosci 28(52):14062–14073
Xu YQ, Jin SJ, Liu N, Li YX, Zheng J, Ma L, Du J, Zhou R, Zhao CJ, Niu Y (2014) Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochem Biophys Res Commun 451(4):568–573
Chen SX, Liao GJ, Yao PW, Wang SK, Li YY, Zeng WA, Liu XG, Zang Y (2018) Calpain-2 regulates TNF-α expression associated with neuropathic pain following motor nerve injury. Neuroscience 376:142–151
Kim SH, Kim DS, Sung YY, Kim HK (2016) Suppression of airway inflammation by Illicium verum and trans-anethole. Planta Med 81(S 01):S1
Kim KY, Lee HS, Seol GH (2017) Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomed Pharmacother 91:925
Vanderwall AG, Noor S, Sun MS, Sanchez JE, Yang XO, Jantzie LL, Mellios N, Milligan ED (2018) Effects of spinal non-viral interleukin-10 gene therapy formulated with d-mannose in neuropathic interleukin-10 deficient mice: Behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain Behav Immun 69:91–112
Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T (1999) Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem 72(4):1466
Navarro A, Saldaña MT, Pérez C, Torrades S, Rejas J (2011) A cost-consequences analysis of the effect of pregabalin in the treatment of peripheral neuropathic pain in routine medical practice in primary care settings. BMC Neurol 11(1):1–11
Sałat K, Gdulaargasińska J, Malikowska N, Podkowa A, Lipkowska A, Librowski T (2016) Effect of pregabalin on contextual memory deficits and inflammatory state-related protein expression in streptozotocin-induced diabetic mice. Naunyn-Schmiedeberg’s Arch Pharmacol 389(6):613–623
Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grant No. 81360182), the Key Research and Development Project in Ningxia Hui Autonomous Region (2017BY079) and the “13th Five-Year Plan” Major Science and Technology Project in Ningxia Hui Autonomous Region (2016BZ07). We are indebted to the staff in the Animal Center and the Science and Technology Centre who provided assistances in the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures were performed as approved by the Institutional Animal Ethics Committee of Ningxia Medical University. This study complied with the IASP guidelines and ethical regulations on conscious-experimental animals research.
Rights and permissions
About this article
Cite this article
Wang, B., Zhang, G., Yang, M. et al. Neuroprotective Effect of Anethole Against Neuropathic Pain Induced by Chronic Constriction Injury of the Sciatic Nerve in Mice. Neurochem Res 43, 2404–2422 (2018). https://doi.org/10.1007/s11064-018-2668-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2668-7