Abstract
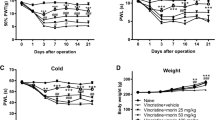
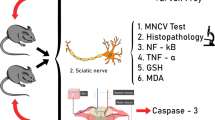
Chemotherapy drugs such as vincristine (VCR) can cause neuropathic pain, and there is still lack of ideal strategy to treat it. The current study was designed to investigate effect of matrine (MT) on VCR-induced neuropathic pain in animal model. VCR (75 μg/kg, i.p. for 10 consecutive days) was administered to induce painful neuropathy model in mice. MT (15, 30 and 60 mg/kg, i.p.) and pregabalin (10 mg/kg, i.p.) were administered for 11 consecutive days. Various tests were performed to assess the degree of pain at different days (1, 6, 11, 16, and 21). Von Frey hair, hot plate, cold-plate and paw pressure tests were conducted to assess the degree of mechanical allodynia, thermal hyperalgesia, cold allodynia and mechanical hyperalgesia in the hind paw respectively. The electrophysiological and histopathological changes were also analyzed. Furthermore, tissue malondialdehyde (MDA), total antioxidant capacity (T-AOC),superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total calcium (TCA), myeloperoxidase (MPO), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10) were measured to investigate possible involvement of MT in inflammation and oxidative stress. Administration of MT attenuated the VCR-induced behavioral alterations as well as electrophysiological and histopathological changes in a dose dependent manner. Further, MT also attenuated the VCR-induced oxidative stress (MDA, T-AOC, GSH-Px, SOD and TCA) and inflammation (MPO, TNF-α, IL-6 and IL-10). Taken together, MT ameliorated VCR-induced painful neuropathy, which might be attributed to neuroprotective effects by subsequent reduction in oxidative stress and anti-inflammatory actions.






Similar content being viewed by others
References
Breivik H et al (2006) Survey of chronic pain in Europe: prevalence impact on daily life treatment. Eur J Pain 10(4):287–333
Bouhassira D et al (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136(3):380–387
Woolf CJ, Mannion RJ (1999) Neuropathic pain: a etiology, symptoms, mechanisms, and management. The Lancet 353:1959–1964
Jensen TS et al (2001) The clinical picture of neuropathic pain. Eur J Pharmacol 429(1–3):1–11
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288(5472):1765–1769
Jaggi AS, Singh N (2012) Mechanisms in cancer-chemotherapeutic drugs induced peripheral neuropathy. Toxicology 291(1–3):1–9
Greeshma N et al (2015) Tetrahydrocurcumin exerts protective effect on vincristine induced neuropathy: behavioral, biochemical, neurophysiological and histological evidence. Chem Biol Interact 238:118–128
Dworkin RH et al (2010) Impact of post-herpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J Pain 11(4):360–368
Sałat K et al (2016) Effect of pregabalin on contextual memory deficits and inflammatory state-related protein expression in streptozotocin-induced diabetic mice. Naunyn Schmiedebergs Arch Pharmacol 389(6):613–623
Quintans J et al (2014) Natural products evaluated in neuropathic pain models: a systematic review. Basic Clin Pharmacol Toxicol 114(6):442–450
Zareba G (2009) Phytotherapy for pain relief. Drugs Today 45(6):445–467
Xu YQ et al (2014) Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B. Biochem Biophys Res Commun 451 (4):568–573
Zhao P et al (2015) Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med 36(3):633–644
Zhang H et al (2013) Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem Toxicol 55:70–77
Kim HK et al (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111(1–2):116–124
Novellis V (2007) Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res 55(2):158–166
Zhao X et al (2015) Antinociceptive effects of fisetin against diabetic neuropathic pain in mice: engagement of antioxidant mechanisms and spinal GABAA receptors. Pharmacol Res 102:286–297
Sircuta C et al (2016) Correlation between the increased release of catecholamines evoked by local anesthetics and their analgesic and adverse effects: role of K+ channel inhibition. Brain Res Bull 124:21–26
Sun T et al (2012) Small interfering RNA-mediated knockdown of NF-κBp65 attenuates neuropathic pain following peripheral nerve injury in rats. Eur J Pharmacol 682(1–3):79–85
Xu M et al (2012) Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain Res 1454:48–64
Hu Z et al (1996) Effects of inhibitor of protein kinase C on brain edema formation evoked by experimental cerebral ischemia in gerbils and rats. Yao Xue Xue Bao 31(12):886–890
Yu J et al (2014) Matrine improved the function of heart failure in rats via inhibiting apoptosis and blocking β3 adrenoreceptor/endothelial nitric oxide synthase pathway. Mol Med Rep 10(6):3199–3204
Wang H et al (2013) Antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury. Pharm Biol 51(7):844–850
Authier N et al (1999) Pain related behaviour during vincristine-induced neuropathy in rats. Neurorep 10(5):965–968
Pourmohammadi N et al (2012) Lithium attenuates peripheral neuropathy induced by paclitaxel in rats. Basic Clin Pharmacol Toxicol 110(3):231–237
Chaplan SR et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Jasmin L et al (1998) The cold plate as a test of nociceptive behaviors: description and application to the study of chronic neuropathic and inflammatory pain models. Pain 75(2–3):367–382
Borta A, Schwarting RK (2005) Inhibitory avoidance, pain reactivity, and plus-maze behavior in wistar rats with high versus low rearing activity. Physiol Behav 84(3):387–396
Beyreuther BK et al (2007) Antihyperalgesic efficacy of lacosamide in a rat model for muscle pain induced by TNF. Neuropharmacology 52(5):1312–1317
Kayser V et al (2003) Effects of the novel analgesic, cizolirtine, in a rat model of neuropathic pain. Pain 104(1–2):169–177
Bain JR et al (1989) Functional evaluation of completesciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83(1):129–136
De Medinaceli L et al (1982) An index of the functional condition of rats sciatic nerve based on measurements made from walking tracks. Exp Neurol 77(3):634–643
Ja’afer F et al (2006) Vincristine-induced neuropathy in rat: electrophysiological and histological study. Exp Brain Res 173(2):334–345
Kandhare A et al (2012) Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 83(4):650–659
Sudoh Y et al (2004) Neurologic and histopathologic evaluation after high volume intrathecal amitriptyline. Reg Anesth Pain Med 29(5):434–440
Villani F et al (2008) Serum cytokine in response to chemo-radiotherapy for Hodgkin’s disease. Tumori 94(6):803–808
Lynch JJ et al (2004) Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy induced neuropathic pain model. Pain 110(1–2):56–63
Diouf B et al (2015) Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 313(8):815–823
Mei L et al (2015) Pharmacogenetics predictive of response and toxicity in acute lymphoblastic leukemia therapy. Blood Rev 29(4):243–249
Muthuraman A et al (2008) Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol 587(1–3):104–111
Muthuraman A et al (2010) Development of animal model for vasculatic neuropathy: induction by ischemic-reperfusion in the rat femoral artery. J Neurosci Methods 186(2):215–221
Shi GB et al (2015) Antinociceptive activity of astragaloside IV in the animal model of chronic constriction injury. Behav Pharmacol 26(5):436–446
Geis C et al (2011) Lacosamide has protective disease modifying properties in experimental vincristine neuropathy. Neuropharmacology 61(4):600–607
Djouhri L, Lawson SN (2004) Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev 46(2):131–145
Kiguchi N et al (2008) The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol 592(1–3):87–92
Milligan ED et al (2005) Controlling pathological pain by adeno virally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21(8):2136–2148
Milligan ED et al (2005) Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 1:9
Uceyler N et al (2006) Heterozygous P0 deficiency protects mice from vincristine-induced polyneuropathy. J Neurosci Res 84(1):37–46
Muthuraman A, Singh N (2011) Attenuating effect of hydroalcoholic extract of Acorus calamus in vincristine-induced painful neuropathy in rats. J Nat Med 65(3–4):480–487
Young W (1992) Role of calcium in central nervous system injuries. J Neurotrauma 9(suppl 1):S9–S25
Muthuraman A, Singh N (2011) Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: an evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complement Altern Med 11:24
Jain V et al (2009) Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol Res 59(6):385–392
Thiagarajan VR et al (2014) Ameliorative effect of Vernonia cinerea in vincristineinduced painful neuropathy in rats. Toxicol Ind Health 30(9):794–805
Luo T et al (2015) Matrine inhibits mouse sperm function by reducing sperm [Ca2+] and phospho-ERK1/2. Cell Physiol Biochem 35(1):374–385
Babu A et al (2015) Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm Biol 53(6):838–848
Zimmermann C et al (2004) Antioxidant status in acute stroke patients and patients at stroke risk. Eur Neurol 51(3):157–161
Chen H et al (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14(8):1505–1517
Pop-Busui R et al (2002) Dissection of metabolic, vascular, and nerve conduction interrelationships in experimental diabetic neuropathy by cyclo-oxygenase inhibition and acetyl-l-carnitine administration. Diabetes 51(8):2619–2628
Mika J et al (2013) Importance of glial activation in neuropathic pain. Eur J Pharmacol 716(1–3):106–119
Tao L et al (2016) Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int Immunopharmacol 34:165–172
Sommer C (1999) Animal studies on neuropathic pain: the role of cytokines and cytokine receptors in pathogenesis and therapy. Schmerz 13(5):315–323
Oft M (2014) IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res 2(3):194–199
Sharma R et al (2013) Association of IL-6, IL-10, and TNF-alpha gene polymorphism with malnutrition inflammation syndrome and survival among end stage renal disease patients. J Interferon Cytokine Res 33(7):384–391
Liou CJ et al (2016) Matrine attenuates COX-2 and ICAM-1 expressions in human lung epithelial cells and prevents acute lung injury in LPS-induced mice. Mediat Inflamm 2016:3630485
Navarro A et al (2011) A cost-consequences analysis of the effect of pregabalin in the treatment of peripheral neuropathic pain in routine medical practice in primary care settings. BMC Neurol 11:7
Vasudevan D et al (2014) Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. Ann Indian Acad Neurol 17(1):19–24
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 81360182), the Ningxia Hui Autonomous Region Science and Technology Support Program (2015BAK45B01), the International Cooperation of Ningxia Scientific Research Projects (2013) and the Key Scientific Research Projects of Ningxia Health Department (20130X). We are indebted to the staff in the Animal Center and the Science and Technology Centre who provided assistance in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The experiments were performed as approved by the Institutional Animal Ethics Committee of Ningxia Medical University. This study complied with the internationally accredited guidelines and ethical regulations on animal research.
Additional information
Shuai-Shuai Gong and Yu-Xiang Li have equally contributed in this work.
Rights and permissions
About this article
Cite this article
Gong, SS., Li, YX., Zhang, MT. et al. Neuroprotective Effect of Matrine in Mouse Model of Vincristine-Induced Neuropathic Pain. Neurochem Res 41, 3147–3159 (2016). https://doi.org/10.1007/s11064-016-2040-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2040-8