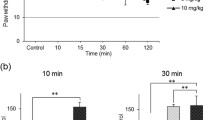
Abstract
Lidocaine effects in the spinal cord have been extensively investigated over the years. Although the intrathecal route is usually used to treat insults occurring in the spinal cord, the local delivery drug via intraparenchymal infusions has gained increasing favor for the treatment of some neurodegenerative disorders. The aim of the present study was to evaluate the behavioral and tissue effects of the intraparenchymal injection of different concentrations of lidocaine into the rat cervical spinal cord. Young male Sprague–Dawley rats were intraparenchymally injected with 0.5%, 1% or 2% lidocaine at the C5 segment of the spinal cord. Other rats were injected with saline solution (sham group). Hot plate test was determined at 0, 1, 2, 3, 7 and 14 post-injection (pi) days. Rats of each experimental group were euthanized either at 1, 2, 3, 7 or 14 pi days. Intact animals were used as controls. Sections of the C5 segment were used for histological, immunohistochemical or immunofluorescence analysis. Injection of 0.5% lidocaine did not affect neuronal counting, did not evoke an inflammatory reaction, nor induce astrocyte activation. Therefore, a concentration of 0.5% lidocaine is suggested to promote anti-inflammatory effects after injury.



Similar content being viewed by others
References
Turtle JD, Strain MM, Aceves M, Huang Y-J, Reynolds JA, Hook MA, Grau JW (2017) Pain input impairs recovery after spinal cord injury: treatment with lidocaine. J Neurotrauma 34(6):1200–1208. https://doi.org/10.1089/neu.2016.4778
Bosshard SC, Grandjean J, Schroeter A, Baltes C, Zeilhofer HU, Rudin M (2012) Hyperalgesia by low doses of the local anesthetic lidocaine involves cannabinoid signaling: an fMRI study in mice. Pain 153(7):1450–1458. https://doi.org/10.1016/j.pain.2012.04.001
Estebe J-P (2017) Intravenous lidocaine. Best Pract Res Clin Anaesthesiol 31(4):513–521. https://doi.org/10.1016/j.bpa.2017.05.005
Kobrine AI, Evans DE, LeGrys DC, Yaffe LJ, Bradley ME (1984) Effect of intravenous lidocaine on experimental spinal cord injury. J Neurosurg 60:595–601. https://doi.org/10.3171/jns.1984.60.3.0595
Cole DJ, Shapiro HM, Drummond JC, Zivin JA (1989) Halothane, fentanyl/nitrous oxide, and spinal lidocaine protect against spinal cord injury in the rat. Anesthesiology 70:967–972
Olschewski A, Schnoebel-Ehehalt R, Li Y, Tang B, Bräu ME, Wolff M (2009) Mexiletine and lidocaine suppress the excitability of dorsal horn neurons. Anesth Analg 109:258–264. https://doi.org/10.1213/ane.0b013e3181a3d5d8
Tobe M, Obata H, Suto T, Yokoo H, Nakazato Y, Tabata Y, Saito S (2010) Long-term effect of sciatic nerve block with slow-release lidocaine in a rat model of postoperative pain. Anesthesiology 112:1473–1481. https://doi.org/10.1097/ALN.0b013e3181d4f66f
Cheng K-I, Lai C-S, Wang F-Y, Wang H-C, Chang L-L, Ho S-T, Tsai H-P, Kwan A-L (2011) Intrathecal lidocaine pretreatment attenuates immediate neuropathic pain by modulating Nav1.3 expression and decreasing spinal microglial activation. BMC Neurol 11:71. https://doi.org/10.1186/1471-2377-11-71
Ineichen BV, Schnell L, Gullo M, Kaiser J, Schneider MP, Mosberger AC, Good N, Linnebank M, Schaw MB (2016) Direct, long-term intrathecal applications of therapeutics to the rodents CNS. Nat Protoc 12:104–131. https://doi.org/10.1038/nprot.2016.151
Vazquez LC, Hagel E, Willenberg BJ, Dai W, Casanova F, Batich CD, Sarntinoranont M (2012) Polymer-coated cannulas for the reduction of backflow during intraparenchymal infusions. J Mater Sci Mater Med 23(8):2037–2046. https://doi.org/10.1007/s10856-012-4652-0
Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, Rothstein JD (2007) Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res 1185:256–265. https://doi.org/10.1016/j.brainres.2007.09.034
Rogawski MA (2009) Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 6(2):344–351. https://doi.org/10.1016/j.nurt.2009.01.017
Federici T, Hurtig CV, Burks KL, Riley JP, Krishna V, Miller BA, Sribnick EA, Miller JH, Grin N, Lamanna JJ, Boulis NM (2012) Surgical technique for spinal cord delivery of therapies: demonstration of procedure in gottingen minipigs. J Vis Exp 70:e4371. https://doi.org/10.3791/4371
Nishida F, Zanuzzi CN, Marquez M, Barbeito CG, Portiansky EL (2014) Vertebral trepanation as an alternative method for intraparenchymal delivery of suspensions into the spinal cord. Analecta Vet 34:11–17
Carter RB (1991) Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain 47:211–220
Portiansky EL (2013) Análisis multidimensional de imágenes digitales [online]. http://sedici.unlp.edu.ar/handle/10915/48218. Accessed 23 Aug 2018
Portiansky EL, Barbeito CG, Goya RG, Gimeno EJ, Zuccolilli GO (2004) Morphometry of cervical segments grey matter in the male rat spinal cord. J Neurosci Methods 139:217–229. https://doi.org/10.1016/j.jneumeth.2004.04.031
Littell RC, Henry PR, Ammerman CB (1998) Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 76:1216–1231
Nishida F, Zanuzzi CN, Martínez A, Barbeito CG, Portiansky EL (2015) Functional and histopathological changes induced by intraparenchymal injection of kainic acid in the rat cervical spinal cord. Neurotoxicology 49:68–78. https://doi.org/10.1016/j.neuro.2015.05.006
Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C (2008) The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest 118(2):763–776. https://doi.org/10.1172/JCI32751
Treede R-D, Meyer RA, Raja SN, Campbell JN (1995) Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483.3:747–758
Schepers RJ, Ringhamp M (2010) Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev 177–184. https://doi.org/10.1016/j.neubiorev.2009.10.003
Luukko M, Konttinen Y, Kemppinen P, Pertovaara A (1994) Influence of various experimental parameters on the incidence of thermal and mechanical hyperalgesia induced by a constriction mononeuropathy of the sciatic nerve in lightly anesthetized rats. Exp Neurol 128(1):143–154. https://doi.org/10.1006/exnr.1994.1122
Jejurikar SS, Welling TH, Zelenock JA, Gordon D, Burkel WE, Carlson BM, Messina LM (1997) Induction of angiogenesis by lidocaine and basic fibroblast growth factor: a model for in vivo retroviral-mediated gene therapy. J Surg Res 67:137–146. https://doi.org/10.1006/jsre.1996.4989
Lei B, Cottrell JE, Kass IS (2001) Neuroprotective effect of low-dose lidocaine in a rat model of transient focal cerebral ischemia. Anesthesiology 95:445–451
Lenfant F, Lahet J-J, Chaillot B, Freysz M (2004) The protective effects of lidocaine on human erythrocytes stored for seven days at 04 degrees C. Cell Mol Biol Lett 9:301–304
Quinteros Villarruel E, Orman B, Borda E (2014) Lidocaine-induced cell growth of human gingival fibroblasts. Role of Na+-K+-ATPase and PKC activities. Pharmacol Pharmacy 5:796–805. https://doi.org/10.4236/pp.2014.58090
Portiansky EL, González PH (1995) Protective effect of lidocaine in the experimental foot-and-mouth disease pancreatitis. Experientia 51:1060–1062
Quinteros Villaruel E, Borda E, Sterin-Borda L, Orman B (2011) Lidocaine-induced apoptosis of gingival fibroblasts: participation of cAMP and PKC activity. Cell Biol Int 35:783–788. https://doi.org/10.1042/CBI20100200
Hollmann MW, Durieux ME (2000) Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93:858–875
Gallos G, Jones DR, Nasr SH, Emala CW, Lee HT (2004) Local anesthetics reduce mortality and protect against renal and hepatic dysfunction in murine septic peritonitis. Anesthesiology 101:902–911
Haim LB, Carrillo-de Sauvage MA, Ceyzériat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278. https://doi.org/10.3389/fncel.2015.00278
Li G, Cao Y, Shen F, Wang Y, Bai L, Guo W, Bi Y, Lv G, Fan Z (2016) Mdivi-1 inhibits astrocyte activation and astroglial scar formation and enhances axonal regeneration after spinal cord injury in rats. Front Cell Neurosci 10:241. https://doi.org/10.3389/fncel.2016.00241
Jegliński W, Koczyk D, Zaremba M, Oderfeld-Nowak B (1995) Bilateral gliosis in unilaterally lesioned septohippocampal system: changes in GFAP immunoreactivity and content. J Neurosci Res 15:41:394–402. https://doi.org/10.1002/jnr.490410312
Su D, Gu Y, Wang Z, Wang X (2010) Lidocaine attenuates proinflammatory cytokine production induced by extracellular adenosine triphosphate in cultured rat microglia. Anesth Analg 111:768–774. https://doi.org/10.1213/ANE.0b013e3181e9e897
Chiu KM, Lu CW, Lee MY, Wang MJ, Lin TY, Wang SJ (2016) Neuroprotective and anti-inflammatory effects of lidocaine in kainic acid-injected rats. Neuroreport 27:501–507. https://doi.org/10.1097/WNR.0000000000000570
Chen JJ, Lue JH, Lin LH, Huang CT, Chiang RP, Chen CL, Tsai YJ (2010) Effects of pre-emptive drug treatment on astrocyte activation in the cuneate nucleus following rat median nerve injury. Pain 148:158–166. https://doi.org/10.1016/j.pain.2009.11.004
Kim DD, Asif A, Kataria S (2016) Presentation of neurolytic effect of 10% lidocaine after perineural ultrasound guided injection of a canine sciatic nerve: a pilot study. Korean J Pain 29:158–163. https://doi.org/10.3344/kjp.2016.29.3.158
Johnson ME, Saenz JA, DaSilva AD, Uhl CB, Gores JG (2002) Effect of local anesthetic on neuronal cytoplasmic calcium and plasma membrane lysis (necrosis) in a cell culture model. Anesthesiology 97:1466–1476
Zheng X, Chen F, Zheng T, Huang F, Chen J, Tu W (2016) Amitriptyline activates TrkA to aid neuronal growth and attenuate anesthesia-induced neurodegeneration in rat dorsal root ganglion neurons. Medicine (Baltimore) 95(18):e3559. https://doi.org/10.1097/MD.0000000000003559
Cherng C-H, Wong C-S, Wu C-T, Yeh C-C (2011) Glutamate release and neurologic impairment after intrathecal administration of lidocaine and bupivacaine in the rat. Reg Anesth Pain Med 36:452–456. https://doi.org/10.1097/AAP.0b013e318228cdb0
Muguruma T, Sakura S, Saito Y (2006) Epidural lidocaine induces dose-dependent neurologic injury in rats. Anesth Analg 103:876–881. https://doi.org/10.1213/01.ane.0000237287.53957.18
Zhao G, Ding X, Guo Y, Chen W (2014) Intrathecal lidocaine neurotoxicity: combination with bupivacaine and ropivacaine and effect of nerve growth factor. Life Sci 112:10–21. https://doi.org/10.1016/j.lfs.2014.07.003
Hampl K, Steinfeldt T, Wulf H (2014) Spinal anesthesia revisited: toxicity of new and old drugs and compounds. Curr Opin Anaesthesiol 27:549–555. https://doi.org/10.1097/ACO.0000000000000108
Ready LB, Plumer MH, Haschke RH, Austin E, Sumi SM (1985) Neurotoxicity of intrathecal local anesthetics in rabbits. Anesthesiology 63:364–370
Funding
This work was supported by the National Agency for Promotion of Science and Technology (ANPCyT) (Grants PICT 2012-0574 to ELP and PICT 2015–2087 to FN). The authors would like to express their sincere gratitude to SAS Institute Inc for providing SAS v.9.3.1 for analyzing the data through an agreement with the College of Agriculture, University of Buenos Aires, Argentina. The expert handling of rats by Mr. H. Enrique is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sisti, M.S., Zanuzzi, C.N., Nishida, F. et al. Effects of an Intraparenchymal Injection of Lidocaine in the Rat Cervical Spinal Cord. Neurochem Res 43, 2072–2080 (2018). https://doi.org/10.1007/s11064-018-2628-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2628-2